DOWNSTREAM PROCESSING FEATURED ARTICLES
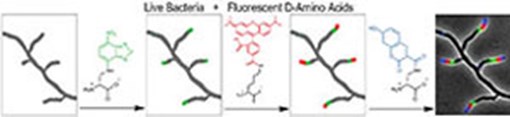 Filming Bacterial Life In Multicolor As A New Diagnostic And Antibiotic Discovery Tool
Filming Bacterial Life In Multicolor As A New Diagnostic And Antibiotic Discovery Tool
An international team of scientists led by Indiana University chemist Michael S. VanNieuwenhze and biologist Yves Brun has discovered a revolutionary new method for coloring the cell wall of bacterial cells to determine how they grow, in turn providing a new, much-needed tool for the development of new antibiotics.
-
Ultrasound Idea: Prototype NIST/CU Bioreactor Evaluates Engineered Tissue While Creating It
Researchers at the National Institute of Standards and Technology (NIST) have developed a prototype bioreactor—a device for culturing cells to create engineered tissues—that both stimulates and evaluates tissue as it grows, mimicking natural processes while eliminating the need to stop periodically to cut up samples for analysis.
-
Cancer Research UK And Lorus To Co-Develop First-Of-Kind Drug, IL-17E, To Treat Solid Tumours
CANCER RESEARCH UK'S Drug Development Office; Cancer Research Technology, the charity's commercial arm; and biopharmaceutical company, Lorus Therapeutics Inc. (Lorus) (TSX:LOR), have partnered to take a new therapy with the potential to treat solid tumours, into its first clinical trial.
-
Next-Generation Kinetex Calculator From Phenomenex Customizes Core-Shell HPLC/UHPLC Method Creation Phenomenex Inc., a global leader in the research and manufacture of advanced technologies for the separation sciences, introduces the next-generation Kinetex Calculator – an online tool that generates customized, optimized Kinetex methods for HPLC or UHPLC systems.
DOWNSTREAM PROCESSING WHITE PAPERS & CASE STUDIES
-
Biomanufacturing Flexibility
This white paper from BIO-G looks at how to create flexible, agile manufacturing facilities to respond to the current glut of biomanufacturing capacity and industry consolidation. Future proofing these facilities for higher titers and multiple product lines is also explored.
-
Automated Bioluminescent ADCC Reporter Bioassay Using Bioengineered Jurkat Cells
Pharmaceutical companies are increasingly exploring new biologic and biosimilar products, and thus increasing monoclonal antibody (mAb) immunotherapeutic research. By Tracy Worzella, Promega Corporation and Brad Larson, Applications Department, BioTek Instruments, Inc.
-
Monoclonal Antibody Purification Platform Using High-Capacity Protein A And Mixed-Mode Chromatography
The first step in purification of an important class of therapeutic proteins, the polyclonal or monoclonal antibodies (mAbs), is their capture from plasma or tissue culture supernatants. Protein A–based media are by far the most common class of affinity products used for this purpose.
-
Compound Profiling And Toxicity
Utilizing a systems approach to drug discovery has generated a multitude of high affinity compounds for various classes of molecular targets with different degrees of disease state validation.
-
White Paper: Computrac® Moisture Analysis Made Simple When people aren’t feeling their best they often turn to medicine for relief from the symptoms that are associated with being ill. The public trusts that the products by pharmaceutical companies will not only make them feel better but will also not have harmful side effects.
-
Biomanufacturing Supply Chains
This white paper examines the major issues with biopharmaceutical supply chains and how to balance inventory and risk optimally in the network.