ABOUT NUCLEUS BIOLOGICS
“Nucleus Biologics, The Cell Performance Company™, is the leading provider of custom cell-growth media and buffer solutions, tools, and technologies for cell and gene therapy. Our mission is to speed the time from scientific discovery to cure by delivering innovative, transparent, and cGMP products and services. From design to delivery, we offer an entire ecosystem of cell culture solutions that seamlessly interface with one another, facilitating easy formulation, configuration, ordering, and manufacture of media and buffers, while addressing the environmental impact of cell culture fulfillment.”
NUCLEUS BIOLOGICS PRODUCTS
-
FGF-2 TOP GMP protein, produced via a sustainable plant-based process, offers a safe, animal-free, and cost-effective solution for cell and gene therapy, enhancing workflow efficiency.
-
Most cryomedia contain toxic DMSO, limiting customization, the QuickStart Media™ platform offers a customizable, DMSO-free cryopreservation option, expediting tailored media development for cell therapies.
-
Discover how a customizable cryopreservation medium with pre-optimized formulas and flexible licensing options provides researchers with a versatile platform for creating tailored solutions.
-
Critical in almost every step of viral vector and gene therapy manufacturing processes as well as fundamental aspects of cell therapy process development, buffers are essential tools enabling optimization of vector stability, transfection efficiency, safety, targeting, and regulatory compliance.
-
NB-KUL 10 is a high-performing QuickStart cryopreservation media that is customizable to meet your process needs. Configure components, concentrations, packaging, and quality.
-
Discover how a customizable, xeno-free solution can optimize HEK-293 cell proliferation and protein expression for gene therapy, eliminating the need for FBS weaning.
-
Scale up your viral vector or gene therapy manufacturing cost-effectively with HEKima Adherent, a QuickStart Media designed to simplify customization and accelerate development and production.
-
Discover a GMP-certified, xeno-free media supplement designed specifically for T cells and stem cells and serves as an alternative to FBS or human serum in traditional media formulations.
-
Dispense cell culture media, buffers, and balanced salt solutions on your benchtop with Krakatoa, the world’s first benchtop media manufacturing system. Utilize compostable powder-filled pods and bring sustainability to your lab while you optimize and iterate your custom formulations. Krakatoa is manufactured by Stoic Bio.
-
Leverage our team of expert cell media scientists to develop, refine, and deliver a complete formula tailor-made to your specifications. A concierge approach to custom media design.
-
Vitronectin XF is a recombinant, extracellular matrix (ECM) that promotes cell proliferation, maintains pluripotency, and supports normal colony morphology for MSCs and iPSCs.
-
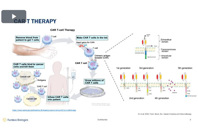
Speed your time to the clinic and create a more potent CAR T-cell therapy with NB-ROC, a xeno-free, serum-free T-cell culture media that can significantly boost transduction efficiency.
-
Bring sustainability to your lab while you optimize and iterate your custom formulations with the world’s first benchtop media manufacturing system.
-
Explore a media specifically designed for the development of CAR-T cell therapy. This media aims to decrease the quantity of viral vectors required, as well as increase the safety of therapeutics.
-
As you transition to the clinic, preparing your cell culture media for regulatory approval becomes a key concern. Support and expedite your transition from development to commercialization with process development services.
APPLICATION NOTES
-
Optimize cell culture for solid tumor research. Replacing traditional serum can significantly enhance CAR-T cytotoxicity, improve consistency, and streamline the path to GMP-ready workflows.
-
Tailor your bioprocess for optimal cell growth, gene expression, and therapeutic production with custom buffers to ensure precise control over pH, ion concentrations, and nutrient availability.
-
This study compared two different cryopreservation mediums and the data highlights which is ideal for preserving the viability of different cell types, including Mesenchymal Stem and HEK-293 Cell Line.
CONTACT INFORMATION
Nucleus Biologics
10929 Technology Place
San Diego, CA 92127
UNITED STATES
Contact: David De Leonardis
FEATURED CONTENT
-
Learn how outsourcing cell culture media and buffers removes bottlenecks, freeing specialized CDMO resources. Ensure consistency, accelerate timelines, and streamline GMP readiness for client success.
-
Media selection is key to CAR-T cell success. Learn how a serum-free, xeno-free medium boosts transduction efficiency, maintains quality, and streamlines the path to clinical scale-up.
-
Generic, off-the-shelf media can introduce hidden costs and delays in cell and gene therapy development. A strategic media approach is a critical advantage that can accelerate your path to the clinic.
-
The quality of non-active ingredients, or excipients, directly impacts product safety and efficacy. Learn why Excipient GMP is vital for cell culture solutions and how it elevates manufacturing standards.
-
The EXCiPACT certification for Excipient GMP quality media and buffers ensures the safety, efficacy, and scalability of cell and gene therapies, accelerating development and improving patient access.
-
Discover how you can revolutionize your stem cell research with a stable, plant-based growth factor that simplifies culture maintenance and boosts cell health.
-
Learn how this game-changing platform simplifies the complex process of custom media design, empowering researchers to expedite their development and achieve optimal results.
-
Cryopreservation enables cell therapy by storing cells long-term. A DMSO-free formula preserves cell viability and health, minimizing toxicities linked with traditional cryoprotectants.
-
Learn about the effectiveness of a xeno-free serum replacement in mesenchymal stem cell (MSC) culture and its potential to enhance cell growth and reduce variability compared to fetal bovine serum.
-
A serum replacement for promoting T cell growth and functionality in cell therapy development shows comparable or superior performance to traditional human serum supplements.
-
Explore the performance of a serum-free medium designed for HEK-293 cells, comparing its efficacy to traditional media for protein expression and cell proliferation.
-
CAR-T cell therapy is evolving rapidly, with scientists exploring new ways to improve efficacy, safety, and applicability. Precision media formulation plays a key role in optimizing therapeutic cell quality.
-
Streamline your AAV gene therapy production process with custom buffers. Learn how optimized formulations can enhance purity, scalability, and regulatory compliance.
-
This study compares the impact of a specific Human Growth Factor Concentrate (hGFC) and fetal bovine serum (FBS) on the growth, expansion, and viability of MSCs.
-
Customizing cell culture media is an important part of the early stages of development considering its composition is crucial for cellular therapeutics' success
-
Compare the use of a cGMP, xeno-free media supplement to human serum as a supplement for T cell growth in cell therapy development.
-
Researchers say turnaround time for custom reagents and media is a large bottleneck for the manufacturing of emerging therapies. Get your therapy to market faster with custom cell culture solutions.
FEATURED VIDEOS
-
Customized formulations, rising costs, and stricter regulatory oversight are transforming bioproduction. Learn how emerging tools can help optimize efficiency, compliance, and cost-effectiveness in media and buffer manufacturing.
-
Learn how optimized, customizable formulations and Excipient GMP manufacturing streamline development, ensuring quality and consistency for a de-risked path to the clinic.
-
A strategic approach to cell culture media is essential for success. Learn how to get a head start toward clinical readiness and avoid future hurdles with optimized media.
-
Reduce risk and speed up timelines as you move towards commercialization. Learn about end-to-end services offering Excipient GMP cell culture solutions for streamlined development and regulatory success.
-
In this presentation, experts emphasize the significance of optimized cell media and buffers in the production of cell and gene therapy products.
-
Discover how Nucleus Biologics can expedite all stages of development from design and optimization, process development scale-up and GMP readiness, and commercial-scale manufacturing.
-
As the cell therapy industry experiences rapid growth, media and reagents present a valuable opportunity to meet future therapeutic needs.
-
Watch to learn about a new serum-free T cell media designed to overcome the barriers hindering CAR-T therapies and explore the importance of transduction efficiency in CAR T-cell therapy.
-
Gain insight into an innovative approach to cell culture media development, and learn about the journey a cell therapy company underwent to accelerate the development of their cell-based therapeutic.
-
Find out how you can simplify the process of media design and customization, reduce cost, and facilitate sustainable science with high quality GMP products.