
DOWNSTREAM PROCESSING

What Bioprocessing Bottlenecks Does Process Intensification Address?
Supply chains, consumables, and data are identified as three of the management challenges that intensified processes and process automation technology (PAT) are addressing at AbbVie and AstraZeneca.

DoE Methodology For Downstream Purification Of pDNA Of IEX Resins
Learn a systematic approach using Design of Experiment to optimize plasmid DNA purification. See how anion exchange resins are evaluated to enhance dynamic binding capacity and process efficiency.
Supporting Development Of mRNA Therapeutics By Enabling Commercial-Scale mRNA Purification
Discover the next generation of scalable affinity capture for high-quality mRNA purification. Learn how a simple, reliable binding mechanism can maximize efficiency and simplify your manufacturing process.

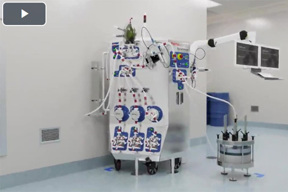
What Is The DynaChrom™ Single-Use Chromatography System?
This chromatography system offers a flexible solution, paired with an advanced software platform, allowing easy integration with downstream process automation.
Considerations In Continuous Chromatography
In this segment of the Bioprocess Online Live event Bioprocess Intensification In The Real World, our presenters discuss considerations for continuous versus batch chromatography and compare notes on the approaches at their respective organizations.

Efficient AAV Purification With AAVx And AAV9 Magnetic Beads
Explore an alternative to chromatographic affinity capture that eliminates the need for universal nuclease treatment by utilizing magnetic beads with ligands specific to AAV.

Expanding Manufacturing Of Purification Resins To Align With Customer Demands
Discover how scaling up processes and building out facilities has allowed for end-to-end solutions that meet customer requirements for both supply and quality in their production lines.

Where Process Intensification Sits In The AbbVie, AstraZeneca Organizations
In this segment of the Bioprocess Online Live event Bioprocess Intensification In The Real World, panelists discuss where and how their roles in setting the course for process intensification sit within their greater organizations.

Speed Up Process Development With HTS Tools For Affinity Chromatography
High Throughput Screening (HTS) chromatography tools can accelerate process development and reduce material usage. Learn about a solution that addresses challenges with their use in new modalities.

Manufacturing Considerations For Viral & Non-Viral Platform Selection
In this webinar, a panel of experts spanning the lentiviral vector, exosome and oncolytic virus fields will discuss the impact of manufacturing considerations on their respective platform selection and ongoing product/process development strategies.

BPI Interviews Purification And Pharma Analytics Leader
Jean Luo, VP of Purification and Pharma Analytics, discusses innovations in bioprocessing, including the development of dPCR assays, lentiviral titer systems, chromatography resins, and supply chain.

Ensuring Quality, Driving Customer Confidence
How does quality assurance help drive scientific progress? A 30-year veteran shares her story of supporting customer needs and the development of groundbreaking new therapeutics.

Driving The Expansion Of mRNA Into The Therapeutic Sphere
Discover the next wave of mRNA therapeutics and how emerging technologies are solving key challenges in downstream processing and analysis to enhance both safety and efficacy.
Optimization Of Anion Exchange Purification For The Large-Scale Production Of Plasmid DNA
In this webinar, we discuss pDNA purification, including how to identify optimal conditions for various chromatography media and optimize recovery and impurity removal.

Ingress Leak Test For Bioprocess Containers
When a visual inspection with magnification isn't enough, the IPA ingress test can definitively confirm a leak. Learn how to perform this critical quality test.

Innovation Of Novel Purification Strategies
In this webinar, we present the development of a novel scavenging resin for clusterin, a persistent CHO host cell protein commonly observed in monoclonal antibody manufacturing processes.

AAV Downstream Challenges: Expert Insights
Gain expert insight into the complexities of AAV purification to learn how industry leaders tackle downstream challenges, optimize workflows, and drive innovation in scalable, efficient gene therapy manufacturing solutions.

Singapore Single-Use Site Capacity Expansion
Thermo Fisher Scientific is expanding our single-use manufacturing network globally, an expansion site closer to Asia Pacific region

IND Prep Versus Clinical/Commercial Scale-Up In Novel Protein Therapeutics
In this segment of the Bioprocess Online Live event Early Process Considerations For Novel Protein Therapeutics, panelists share detailed considerations for scale-up of complex protein therapeutics.
mAb Up- And Downstream Process Intensification Strategies
Discover how to reduce monoclonal antibody (mAb) manufacturing time and costs through process intensification. Learn about continuous perfusion, ultrahigh cell density banks, and more.

An Advanced Single-Use Solution for Downstream Bioprocessing
A single-use chromatography system is offering an innovative solution tailored to meet the needs of evolving downstream purification bioprocesses.

Purification Tools To Address Low Yield Challenges With Protein A
Discover new affinity chromatography resins for engineered modalities. Learn how they boost yield and reduce aggregation with high-specificity binding and mild elution. Ideal for purifying novel mAbs, BsAbs, and mAb fragments.

Process Liquids And Buffers Offering
Whether you are a researcher, scientist, or industry professional, this video will provide valuable insights into our large volume liquids manufacturing capabilities.

Three Novel Protein Therapeutic Worldviews
In this segment of the Bioprocess Online Live event Early Process Considerations For Novel Protein Therapeutics, we meet panelists who share their topline perspectives on developing complex, novel protein therapeutics.

BioTitan Retention Device Overview
Learn about a retention device that provides robust fortification for single-use assemblies. Its 360-degree seal strengthens connections, reduces leak risks, and ensures process integrity for fluid transfer.
Affinity Resins Overview
We have developed a unique portfolio of affinity resins to help you develop biosimilars, biobetters, and other types of recombinant proteins. These affinity resins can be used for clinical and commercial production.

Rethinking Sustainability In Bioprocessing
Discover how sustainability is transforming bioprocessing through innovative single-use technologies that help reduce environmental impact and support responsible, forward-thinking biomanufacturing.

Thermo Fisher Scientific Suzhou, China Manufacturing Site Capabilities
Watch this video to learn more about this manufacturing site capabilities, quality management systems, PPI implementation, product expansion, assurance of supply and lead times.

Advancing Gene Therapy Development With A Multi-Serotype AAV Resin
In this webinar, leading CDMO ABL and Thermo Fisher will share data on implementation of the POROS™ CaptureSelect™ AAVX affinity resin into such an AAV purification platform, including its performance in purifying several serotypes.

Advancing Vaccine Development With Novel Chromatography Solutions & Quality Testing
Learn more on novel chromatography solutions that can help improve the downstream processes of different vaccine types such as mRNA, recombinant proteins, and Virus-Like Particles (VLPs).
