How Distributed Automation And Digital Plant Maturity Support Resilient Biomanufacturing

Embracing digital transformation, when combined with resilient biomanufacturing best practices, supports increased productivity and efficiency in a biomanufacturing environment. From the more traditional data historians, Manufacturing Execution Systems (MES) and Supervisory Control and Data Acquisition (SCADA) systems to today’s advancements in automation, machine learning, smart plants, artificial intelligence (AI) and the Industrial Internet of Things (IIoT)—adoption of digital technology in biopharma has come a long way, especially evident in the last several years. In fact, 49% of biopharma respondents agree that implementing a better digital innovation strategy ranks at the top of their list of priorities, with 82% further confirming that digitalization of operations will continue post-pandemic (1).
While use of the Cloud, AI, data lakes and more will make up future digitalization strategies, increased adoption of controllers and automated systems, especially in biopharma, could position the industry for accelerated drug production timelines and to better address existing bioprocess optimization challenges. Forecasted to grow at a compound annual growth rate (CAGR) of 10% by 2030 (2), use of controllers and automation systems in biopharma “have the potential to lower production and labor cost (by 20-30%), optimize energy consumption (by 5-30%) and increase overall productivity (by 10-20%),” according to the market research from Roots Analysis.
Utilizing data better across the product lifecycle, sometimes referred to as digital maturity, can provide greater visibility into workflows, thus enabling better decision-making and facilitating issue management and resolution. Yet, many biomanufacturers realize that simply utilizing data better is not enough. “Leapfrog digital innovation”—or what Deloitte otherwise defines as making focused digital technology investments that change the status quo in biopharma—can help support transformational change across functional areas and value streams, optimally engage patients and partners, and bring drugs to market faster (1). In addition, by implementing distributed automation and a data historian simultaneously to elevate their position on the Digital Plant Maturity Model (DPMM) scale, users can subsequently move a step closer to more flexible, configurable biomanufacturing environments.
Success depends on flexible, agile manufacturing
One example of changing the biopharma status quo, especially as it relates to drug development timelines, is the pace at which companies were able to bring COVID-19 vaccines to market. Although the effort fell short of its original goal of 300 million doses by January 2021, many leaders viewed the achieved timeline a success, compared to the seven-to-10 years it typically takes to bring a drug to market. Some industry leaders recognized their shortcomings in responding quickly to changes in product type and demand, or in meeting large-scale biomanufacturing demand in short timeframes, and took action to strengthen their resiliency, specifically to provide more flexible, agile manufacturing. But as Adrian Wallis of Lakes BioScience pointed out earlier in 2022, “While the notion of ensuring robust supply chain capability through better considerations around agility is important, there is still a place for just-in-time” manufacturing. Watch the video to hear more insights from Lakes BioScience and Teesside University’s National Horizons Centre on how they address manufacturing and talent needs.
Providing resilient agile manufacturing depends on multiple factors, including available in-country (vs offshored) drug manufacturing capacity and space to support production demand—a key shortcoming many biopharma leaders experienced in the last several years. In fact, 67% of executives foresee biologics manufacturing dramatically increasing in their own countries over the next three years, according to the Biopharma Resilience Index. With that increase in capacity must come the focus on efficient biomanufacturing environments, workflows and processes that allow for a quick, agile response to changes in product type or market demand requirements.
For SanKav Pharmaceuticals, the role and responsibility of managing such biopharma supply chain management aspects comes down to being able to do so under one roof.
“The way we designed SanKav’s facility is to have a FlexFactory™ approach, where we have upstream, downstream and processing adjacent to each other where we’re able to suit different needs of different technologies when we’re using our reactors and filtration systems and utilities,” explained Tushar Gupta, director of engineering and operations at SanKav. “As we’re doing changeovers or taking a new batch or product in-house, we’re able to use the same space to accommodate that product. So, the type of equipment we procure where we can interchange different pieces and create new equipment trains, we’re able to process, package and fulfill those customer demands and release the product to clinical trials or even at the commercial scale.”
And as Gupta confirmed, “To have better adaptability and agility throughout biopharma supply chains requires a teamwork collaboration consisted of real-time communication, data and information sharing.” (Watch the video to hear more insights from SanKav Pharmaceuticals on the importance of available components to biologics production lines, biopharma drug production preparedness, and more.)
Combined historian and distributed automation benefits
One key example of data and information sharing that evolved over the last several decades is that of a data historian. Historically known for time-series information sharing of production and process data, data historians used in today’s biopharma landscape can serve as a steppingstone up the digital plant maturity curve.
Many process engineers would agree. A data historian is not a new concept, given its historical use in industrial applications and SCADA systems. But with industry advancement in IIoT and progression of Industry 5.0—combined with increased adoption of automation—the advantages in biomanufacturing productivity and efficiency that biopharma organizations can gain from such technologies are considerable.
According to Gartner, 85% of infrastructure and operations leaders currently without any full automation will become more automated in the next two to three years. Additionally, 70% of organizations will implement structured automation to deliver flexibility and efficiency,” up from 20% of organizations in 2021 (3).
For those biopharma and biomanufacturing companies willing to take greater risks or innovate faster, assessing their current level of digital adoption or at minimum, considering which technologies can help them advance up the DPMM—whether data historian, automation or an integration of the two—may be a worthy exercise.
Developed in 2016 by the BioPhorum Operations Group, the DPMM describes the stages of digital maturity, “from simple paper-based plants through to the fully automated and integrated ‘adaptive plant’ of the future (4).” As explained in a previous article, biomanufacturers can leverage the framework to identify at which level they fall, address their next steps with regards to the transformation of manufacturing capabilities, and address previously unclear questions around how to improve digital maturity.
To provide that stepping-stone approach to digital plant maturity, an added level 2.5 can now represent the short-term goal of many manufacturers deploying a Distributed Control System (DCS) with central historian as an interim step to becoming a connected plant.
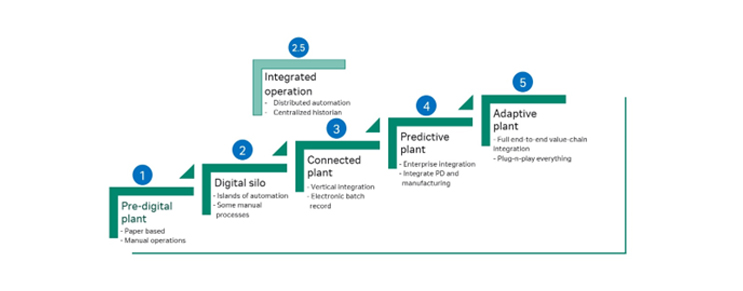
Cytiva Figurate™ automation DCS solutions can help companies reach level 2.5. The FlexFactory™ historian allows users to implement easy-to-use data, alarm, and audit trail aggregation, mitigating the need to “gown up” and manually retrieve critical GMP data from the production suites.
The power of UNICORN™ software on process automation and Design of Experiments
For biosimilars provider Alvotech, having a partner like Cytiva which understands the need to streamline operations and transfer processes throughout the development lifecycle is key.
“The power of Design of Experiments application within UNICORN allows for the retrieval of maximum amounts of information and data from the minimum amount of experiments,” explained Andrew Falconbridge, VP of Process Technology and Innovation at Alvotech. “Very complex experiments can be designed and performed rapidly—in days instead of weeks. This greatly reduces timelines, reaching the required level of understanding of a process or experiment, faster.”
Trusted for 30-plus years by academia and research scientists, UNICORN™ software from Cytiva can control and automate a variety of processes. It takes what is traditionally a very time-consuming and often specialized process and empowers the operators and scientists to spend less time on manual handling of equipment. These capabilities allow more time to perfect processes and explore new target molecules. (Watch the video to hear more insights.)
Elevate your digital biopharma journey
Biomanufacturers face many challenges, with speed to market, risk mitigation, and cost control being at the top of the list. Fortunately, recent advancements in digital automation can help address and overcome these challenges. As manufacturers strive to build digital maturity with enterprise automation, Cytiva automation experts can discuss your specific current and future needs and determine the most appropriate approach for your unique situation.
Download the brochure or contact Cytiva to learn more.
Read Cytiva's previous article, “Demonstrating ROI For Increased Digital Plant Maturity,” to better understand the Total Cost of Ownership (TCO) against planned ROI to achieve a higher level of digital maturity.
References
- Deloitte Development LLC. Biopharma digital transformation: Gain an edge with leapfrog digital innovation. https://www2.deloitte.com/content/dam/Deloitte/it/Documents/life-sciences-health-care/DI_Life_sciences_digital_innovation.pdf. Published 2021. Accessed November 23, 2022.
- Roots Analysis. Bioprocess Controllers and Automation Systems Market By Type of Controllers (Upstream / Downstream Controller System and Bioprocess Control Software), Scale of Operation, Types of Processes Controlled (Cell Cultivation, Microbial Fermentation, Chromatography and Tangential Flow Filtration), Mode of Operation, Compatibility with Bioprocessing Systems, and Key Geographical Regions (North America, Europe, Asia-Pacific and Rest of the World): Industry Trends and Global Forecasts, 2021-2030. https://www.rootsanalysis.com/reports/bioprocess-controllers-and-automation-systems-market.html. Published 2021. Accessed November 23, 2022.
- Gartner Inc. Gartner Survey Finds 85% of Infrastructure and Operations Leaders Without Full Automation Expect to Increase Automation Within Three Years. https://www.gartner.com/en/newsroom/press-releases/2022-10-03-gartner-survey-finds-85-percent-of-infrastructure-and-operations-leaders-without-full-automation-expect-to-increase-automation-within-three-years. Published October 3, 2022. Accessed November 23, 2022.
- BioPhorum Operations Group. The development of a Digital Plant Maturity Model to aid transformation in biopharmaceutical manufacturing. https://www.biophorum.com/wp-content/uploads/bp_downloads/Digtal-Plant-Maturity-Model-White-Paper.pdf. Published 2017. Accessed November 23, 2022.


