
PROCESS ENGINEERING

Reimagine Sustainability In Bioprocessing
Achieving sustainability in bioprocessing labs presents distinct challenges. Learn how to reduce your environmental footprint while maintaining efficiency and avoiding extensive re-validation efforts.

Accelerating Novel Cell Therapies To Market
Learn about two matrices that, when combined, provide an optimal solution for the process development and manufacturing of novel induced pluripotent stem cell therapies.

Collaboration & Expertise, The Keys To Efficiency And Scalability Across All Modalities
View this video and discover our global vaccine capabilities from process development to full-scale GMP-manufacturing and in all modalities.

OsmoTECH® HT Automated Micro-Osmometer
The automation-friendly OsmoTECH HT, the only plate-based micro-osmometer, is designed in a 96 well format that ensures efficiency and acceleration of osmolality testing in early upstream process development, formulation development and stability testing.
Enhanced API Solubility And Stable, High Drug Loads
Poor solubility of APIs is a critical challenge in drug development. One formulation technique to address this challenge is hot melt extrusion.

Nanoassemblr Blaze mRNA Vaccine Demo
NxGen Technology delivers a scalable means to perform the critical particle formation step in a nanomedicine development process.

CDMO Hunger Games And The State Of Biotech With Siren Biotechnology's Nicole Paulk, Ph.D.
Biotech leader Nicole Paulk, Ph.D., joins “Better Biopharma” to discuss Siren Biotechnology’s switch from plasmids to producer cell lines, CGT funding challenges, and the competition that determined which CDMO won Siren’s busi...

Parteck® SRP 80 - Reliable Sustained Drug Delivery
Parteck® SRP 80, a functional excipient based on polyvinyl alcohol (PVA), provides reliable sustained drug delivery over long release periods.

Meet The Experts: Part 2 - Raman Spectroscopy
Watch part 2 of this 4-part interview series to explore Raman spectroscopy, one of the main PAT tools for monitoring bioprocess CQAs and CPPs, with expert Fabien Caron.

How To Choose A Capping Method In MRNA Production
Is your mRNA production process ready for scale up? Learn how to choose a capping method to optimize your mRNA yield and process costs.

Navigating The Workflow From Process Development To GMP Manufacturing For Making Clonal iPSC-Derived Master Cell Banks
In this video, we present data for optimizing choices of which combinations of GMP-compatible matrix, dissociation reagents, and media can best be employed for single cell cloning of iPSCs in process development.

Engineering Bacterial Vector-Based Immunotherapies With OS Therapies' Paul Romness and Robert Petit, Ph.D.
In this episode of “Better Biopharma,” host Tyler Menichiello is joined by OS Therapies’ CEO, Paul Romness, and chief medical and scientific officer, Robert Petit, Ph.D. The three discuss the development and manufacturing of the com...

Small-Scale Oligonucleotide Synthesizer That Supports Scale Up
Learn about a small oligonucleotide synthesizer with big impact that features a user-friendly interface, compact footprint, and more. This small scale system is user friendly and supports scale up.

Developing Antibodies To Block Neuroinflammation With MindImmune's Stevin Zorn, Ph.D.
In this episode of "Better Biopharma," host Tyler Menichiello is joined by MindImmune's president and CEO, Stevin Zorn, Ph.D. They discuss the development of the company's lead candidate, MITI-101, a monoclonal antibody designed to prevent peripheral imm...

Key Functions Of The ÄKTA Oligosynt Synthesizer
Discover a compact, fully automated oligonucleotide synthesizer for robust, scalable process development. Learn how it can help establish a robust and reproducible oligo synthesis process.
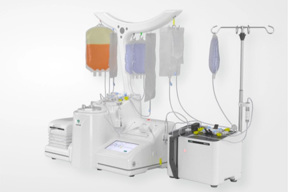
A Closed And Automated Solution For Cell Therapy Manufacturing
Streamline your cell therapy manufacturing process and workflow by automating and closing your cell isolation, cell harvest, and formulation with a system designed to offer you versatility.
How To Best Optimize mRNA Production Workflows
Multiple steps in the mRNA manufacturing process can appear more complex than they have to be. Learn how to simplify your approach and produce the best possible mRNA vaccines and therapeutics.
