Best Practices For Multisite, Concurrent Rollout Of Manufacturing Execution Systems
By Paul Murray, Ph.D., Enterprise System Partners (ESP)

The current expansion in manufacturing execution system (MES) rollouts within the life science industry has led to a number of challenges with regard to multisite concurrent rollouts and how to achieve them in a timely manner. This challenge is compounded by a global lack of experienced MES resources given the niche nature of the projects. Here, we discuss how different standardizations can help to address these challenges.
These standardizations can be broken down into a number of areas:
- Base application standardization
- Business process/master batch record (MBR) harmonization
- Software development life cycle (SDLC) standardization
- Deployment model.
Base Application Standardization
The concept of base application standardization, or a core application, is well established within the life science industry. This typically involves designing and building customer-specific customizations, such as global interfaces (typically enterprise resource planning [ERP]), specific master data configuration (e.g., user security groups/roles) and the qualification of the above. This can then be leveraged on a site-by-site basis, so that only the local business processes (typically master batch records, equipment and material models, level 2 interfaces, if applicable, and local master data) need to be configured and qualified.
However, even with the above, there can still be a wide variation even before the local business processes are modeled. First, guidance should be given on what interfaces should be in scope. (Interfaces are always the weak point in any system integration, and this needs to be carefully managed.) Typically, an ERP interface is taken as a given, and is in widespread use within the industry for order and material control. However, other interfaces need to be evaluated to determine if they bring business benefit or not. In addition, the whole life cycle impact needs to be factored into the cost benefit analysis (i.e., system upgrades). At level 4, interfaces such as laboratory information management systems (LIMS) or electronic document management systems (EDMS) are sometimes seen, while interfaces to computerized maintenance management software (CMMS), learning management software (LMS), and corrective and preventive action (CAPA) software are rarely observed in the industry. This needs to be in a system roadmap and requires a strong governance process in order to be successful.
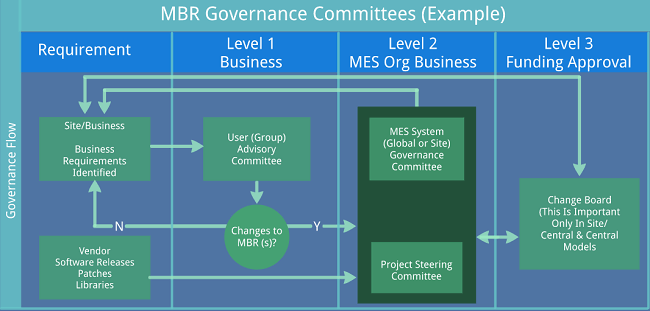
Interfaces to Level 2 systems, while not always seen, are a common occurrence. Here, very careful thought should be given to the functionality of the interfaces and global standards or guidelines set by the organization. As with all interfaces, data exchange should be simplified to the minimal amount needed for the Level 2 system to perform its function (download typically being Recipe ID, Batch ID set points) and read only critical data back to MES. The temptation to tightly integrate MES with automation should be avoided, as it tends to overcomplicate the interface and can cause issues if misalignment between the systems occurs. In the case of multiple Level 2 systems, the use of a data historian can reduce risk and complexity by providing a single interface to MES.
Standardization can be driven further at the core application level by setting standards on naming conventions. This can be particularly beneficial for data exchange with Level 4 systems which tend to be regional or global instances, for example, having global standard conventions for ERP master data (i.e., parameter structure) when used in combination with generic master batch records (GMBRs) in processes such as packaging. However, such standardization will need multi cross-functional stakeholder buy-in (e.g., MES, supply chain, QA) in order to be effective.
Standard system management standard operating procedures (SOPs) can also be created at the global level for reuse among sites, covering such topics as user administration, backup and disaster recovery, and life cycle management.
Business Process Harmonization
Many of the sites that adopt MES will have similar processes. Even if the processes are different from site to site, there will still be aspects that are common, such as material usage, equipment management, IPC checks, etc. In addition, standards on the content of MBRs can also be developed, such as level of instruction text (best practice is to minimize this with detailed process instructions documented in SOPs, which are easier to update and manage than MBRs) and signature requirements (system log on vs. single vs. verification signatures).

Ensure that your product quality doesn't suffer when you rollout at multiple sites. Attend the webinar:
Improving Product Quality During Technical Transfer
In processes where there is a very high degree of commonality, specific global process templates may be developed which then require minimal localization at the site. This tends to be more applicable to processes such as packaging but can still be applied to aspects of other processes such as tablet compression, coating, and fill finish operations. The appropriate use of generic master batch records (GMBRs) will also drive a degree of harmonization across similar product families.
Where a global process template cannot be developed due to diversity, standard building blocks can still be used to help configure the local process around the items mentioned above such as line clearance, IPC testing, etc. These, together with the base application standardization, will still instil a level of harmonization across the organization.
This will help give clear guidelines to sites on how to configure MES to their local business process, while at the same time permitting an acceptable degree of flexibility for local nuances and variations, thus reducing the typical churn on design decisions that can be experienced on MES implementations. However, as mentioned previously, such an approach will only work if it has cross-functional input, buy-in and approval from the key stakeholders, such as QA, manufacturing, supply chain, and technical operations, and is overseen by a strong governance process.
SDLC Standardization
An area where many MES projects run into difficulty is around documentation. While many organizations do have a software development life cycle (SDLC) process that mandates documents to be generated as part of a system implementation, the devil is always in the detail. While we know what documents are needed, what should be their content?
When defining this, organizations should think of all their processes and evaluate the best practice. For example, what may seem suitable for a small process such as packaging may be unmanageable for a large biotech upstream/downstream process.
Having specific template examples around requirements, design/configuration, and validation documents is key to minimizing risk in this area. Furthermore, the use of global guidelines and SOPs around MBR lifecycle management is also extremely beneficial. Such documents typically detail the design documentation requirements, along with what can be verified at design versus what requires a functional test, not only at project initiation but also for ongoing system changes and updates. Where such documents exist, it greatly clarifies the approach to be taken and reduces unnecessary debate and churn on this topic.
However, as mentioned previously, such an approach will only work if it has cross-functional input and buy-in backed up by strong governance.
Deployment: Individual Vs. Multisite?
While all the above are extremely useful tools to assist an efficient multisite rollout, this will not happen without the correct resources and deployment model.
The traditional approach to a multisite rollout of MES systems within the life science industry has been to either have individual site teams perform the implementation themselves or have a global team implementing on a site-by-site basis. Both models can involve a mixture of staff and system integrator resources, and both options have disadvantages in that:
- Implementations by individual site teams are more likely to result in different approaches being taken in design, documentation, and testing, leading to a divergence in processes at a time when many companies are looking to harmonize business processes, and also potentially taking longer to implement.
- Implementations by a global team moving from site to site can mitigate against this, but this model has the disadvantage of extended deployment timelines given the typical size limitations and bandwidth of such global teams.
In order to efficiently implement concurrent multisite rollouts, a new approach is required:
One such approach is the use of a centralized model, whereby a central team can remotely implement projects simultaneously. This has the advantage of facilitating standardization of builds, SDLC, etc., and also for the most efficient use of high value experienced resources across multiple projects.
However, in order for this to succeed, the following are key:
- Strong governance and management
- Clear scope/understanding of requirements
- Clearly defined standards
- Global content configuration and documents
- Best practice use of GMBRs and parameters
- Cross functional buy-in
- Appropriate Integration strategy
- Accountability of all stakeholders
When all the above are combined successfully, an efficient multisite rollout with a high degree of global harmonization can be achieved.
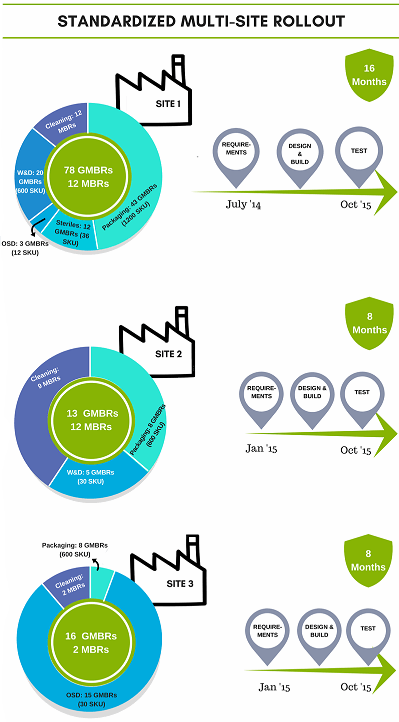
With the appropriate site business resources (i.e., QA, operations and technical staff) involved, with structured design workshops, high value MES technical resources’ time can be used more efficiently. Organizations can leverage their rollouts to their global standards and content while utilizing iterative prototyping. End user engagement is optimized while also benefiting from post go-live support.
As you can see above, a robust and well-governed implementation plan, along with the best possible team in place, can lead to significant time savings and the greatest long-term benefits to operations.
Conclusion
Where traditionally MES was rolled out on an individual site basis, now, as our understanding of MES matures, standardization and harmonization across multiple sites to improve quality and reduce costs are being demanded more. With the correct tools, including strong governance, companies can gain considerable benefits by utilizing this approach.
About the Author:
 Paul Murray, Ph.D., is director of MBR Factory at Enterprise System Partners (ESP). He has specific expertise in manufacturing execution systems (MES), manufacturing information systems (MIS) and laboratory information management systems (LIMS). As director of MBR Factory, he leads ESP’s team of engineers in the delivery of this off-site MBR development methodology for global multisite rollouts for leading biopharmaceutical organizations. He has over 20 years’ experience in the life science industry, including staff positions with Johnson & Johnson and Amgen.
Paul Murray, Ph.D., is director of MBR Factory at Enterprise System Partners (ESP). He has specific expertise in manufacturing execution systems (MES), manufacturing information systems (MIS) and laboratory information management systems (LIMS). As director of MBR Factory, he leads ESP’s team of engineers in the delivery of this off-site MBR development methodology for global multisite rollouts for leading biopharmaceutical organizations. He has over 20 years’ experience in the life science industry, including staff positions with Johnson & Johnson and Amgen.
