
RECENT WEBINARS

Five Practical Considerations To Move From Concept To Clinic
Learn more about five key factors for advancing targeted in vivo LNP programs, from formulation and targeting strategies to bioanalytical readiness and scalability.

Overcoming Regulatory Hurdles In AAV Production
Demonstrating residual reagent clearance is a critical regulatory requirement in AAV manufacturing. Explore how specialized assays and strategic partnerships streamline compliance.
From Lab To Commercialization: Simplifying Bioprocess Scale-Up
Explore how unique cuboid geometries and advanced mixing dynamics create consistent performance from the bench to commercial manufacturing, ensuring flexibility and improved productivity.

Bridging The Gap Between Product Readiness And Equipment Availability
Discover how manual precision at scale bridges packaging gaps, accelerates product launches, and ensures uninterrupted supply to deliver quality when automated equipment isn’t yet operational.

Automating Visual Inspection Qualification
Transferring visual inspection data from paper to digital is essential for Pharma 4.0 compliance. Learn practical strategies to eliminate errors, automate data tracking, and enhance quality assurance.

When A Logbook Project Becomes A Digital Movement
A leading pharma company accelerated execution and compliance in GMP operations. See how modular apps replaced manual processes, setting a new foundation for continuous innovation.
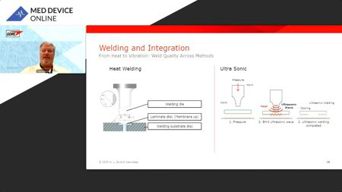
Seal The Deal: Mastering Vent Integration In Medical Devices
Learn how precise vent placement and microfiltration design optimize performance in life science devices. See practical troubleshooting strategies and a real-world case study.

Lessons From FDA 483s And Warning Letters: Cleanroom Compliance
Learn critical lessons from FDA 483 observations and Warning Letters to proactively address common GMP cleanroom compliance failures and build an inspection-ready facility.

Eliminate Risk From Your Viral Vector Tech Transfers
Explore strategies to simplify viral vector tech transfers, reduce risk, and maintain quality under tight timelines, as well as a case study that demonstrates how to streamline this critical process.

Navigating Innovation And Challenges In CGT Bioprocessing
This expert panel breaks down the practical realities of today’s supply chain while forecasting how emerging technologies will redefine quality standards.

Navigating Regulatory Expectations For Injectable Packaging
Explore insights on extractables, focusing on how they relate to the revised EU GMP Annex 1, new and evolved expectations under USP <382>, and the use of VHP decontamination in aseptic environments.

Unlock Productivity In mAb Production: Upstream And Downstream Intensification
Boost your mAb manufacturing efficiency by mastering upstream and downstream intensification strategies. Learn how to minimize cost and time while increasing overall productivity.

Critical Path For Gene Therapy: AAV Analytical Lifecycle Considerations
Explore considerations for phase-appropriate AAV characterization and release activities from pre-clinical to late-phase products. Review validation challenges and paths for maturation of analytics.

Raising The Bar In Gene Therapy
Learn how a science-driven and digitally structured approach reduced onboarding timelines for gene therapy from 12 months to just 3, setting a new benchmark in technology transfers.
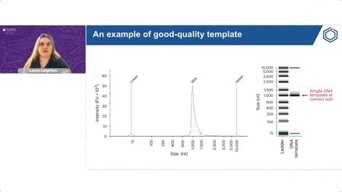
The mRNA Journey: From Design To LNP-Ready Molecules For Research
Learn a validated, end-to-end protocol for producing high-quality in vitro transcription (IVT) mRNA for preclinical research. Find out how to optimize sequence design, small-scale synthesis, and more.
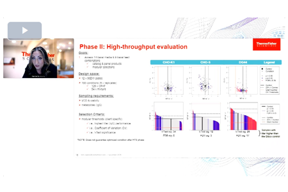
Smart Media Screening And Design: Saving Time And Boosting Titers
Optimizing your cell culture media selection is crucial for clinical advancement. Learn how high throughput screening techniques accelerate media and feed optimization to significantly boost your biologics' titers.

Streamlining pDNA: Capacity, Complexity, And Cutting-Edge Solutions
Learn how flexible facility design and process innovation are accelerating pDNA manufacturing, as well as key strategies to avoid scale-up pitfalls and meet growing therapeutic demand.

Are You Keeping Pace With Oligo Synthesis Optimization?
The rapidly expanding oligo market demands optimized RNA synthesis. Examine key innovations and workflow insights to maximize resources and control long-term manufacturing costs.

From Readiness To Results: Unlocking Capacity For Expanding Product Demand
Learn the six pillars that bridge operational readiness and operational excellence. Discover a proven framework to build a resilient, high-performing manufacturing operation and unlock necessary capacity.

Accelerate Your pDNA And mRNA Process Development
Explore a structured approach to optimizing and scaling plasmid DNA production using E. coli, with insights into key growth parameters, benchtop feasibility, and pilot-scale performance.

Driving Robust Tech Transfer In Biologics: CFD Simulation
Computational Fluid Dynamics simulation is essential for robust biologics tech transfer. See how this predictive tool optimizes critical scale-up processes, reducing risk and expediting time to market.

Practical Solutions For Protein Analytics And Residual DNA Testing
Discover practical solutions for common quality control bottlenecks in biotherapy manufacturing. Learn how to improve protein analytics and DNA testing robustness and accelerate time to market.
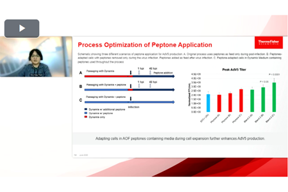
Boosting Vaccine Yields Through Smarter Cell Culture Media Optimization
Unlock efficient vaccine production by understanding how tailored peptone supplementation optimizes cell culture media, improving cell growth and overall yield across multiple critical cell lines.

Navigating Commercial Cell Therapy Manufacturing
Explore expert insights on manufacturing strategies, quality management, scalable production, and digital tools that enhance collaboration and ensure consistent, efficient multi-product facility operations.

Driving Sustainability In Bioprocessing With Biobased Single-Use Solutions
Discover how biobased materials in bioprocessing enable measurable carbon emission reductions. Learn practical strategies to meet decarbonization goals and adopt greener, operationally sound practices.

Achieve Pharma 4.0 With Next Generation Software-Defined Manufacturing
Explore how software-defined manufacturing is helping companies overcome legacy system challenges, improve OEE by up to 25%, and accelerate batch release cycles while maintaining compliance.

Enhancing Viral Vector Sterile Filtration: Process Efficiency And GMP Alignment
Explore filtration strategies that improve viral vector yield and quality, with insights on AAV and lentivirus workflows, plus practical guidance for implementing PUPSIT in line with requirements.

Faster Tech Transfers Through Seamless Digital Transformation
Manual process documentation slows innovation. Learn how modular libraries and digital connectivity enable faster, error-resistant execution, bridging the gap between process design and automation.

Developing Your Risk-Based Approach To Single-Use System Integrity
Explore how a holistic approach to single-use system integrity can strengthen contamination control, improve operational efficiency, and meet evolving regulatory expectations in manufacturing.

Optimize DNA Clearance In High-Salt, GMP-Grade Purification Processes
Explore how salt-active endonucleases overcome DNA clearance challenges in viral vector purification by digesting down to 3–5 nucleotides and performing reliably in high-salt conditions.
