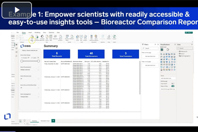
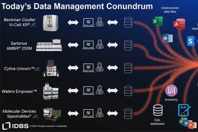
ABOUT IDBS
IDBS provides purpose-built software solutions to address the data management challenges prominent across the BioPharma lifecycle and supply chain.
Leveraging more than 30 years of experience in life science informatics, IDBS is uniquely positioned to deliver a portfolio of innovative BioPharma knowledge management technologies to streamline the capture, analysis, reporting and sharing of data required to accelerate the next generation of life-changing therapies.
In addition to our signature E-WorkBook software, IDBS introduces the industry’s first BioPharma Lifecycle Management (BPLM) solutions. Cloud-native and analytics-centric, IDBS Polar and Skyland PIMS platforms enable customers in research, development and manufacturing to efficiently and compliantly access critical data and insights to make faster, smarter decisions with greater confidence.
WEBINARS
-
Struggling with data integrity challenges? Discover proven strategies for GxP compliance and inspection readiness in this essential webinar. Learn to build a robust framework and avoid common pitfalls.
-
The panel explores what the next generation of digital technology will look and feel like from users’ perspective. Hear from experts at Microsoft, GSK, TetraScience, Emerson and IDBS.
-
Learn how to select and manage trusted software suppliers to streamline your digital transformation, mitigate risks, and ensure regulatory adherence. Watch to discover best practices and real-world examples.
-
How can organizations establish the foundation for advanced analytics, AI and ML, in pursuit of a robust and adaptive lab of the future?
-
Explore the challenges of cloud migration in the BioPharma industry and discover the keys to ensuring a secure, reliable, efficient, affordable, and sustainable cloud operation.
-
This presentation explores how many organizations have not fully utilized digital and analytics tools, leading to inefficiencies in data management and delays in delivering therapeutics to market.
-
Unlock the full potential of AI and digital solutions with these practical steps to begin moving your BioPharma organization's goals forward with optimized data usage.
-
In this presentation we discuss the importance of embracing digital solutions, the paradigm shift toward finding data solutions, and the terminology used by digital transformation leaders.
-
Learn how you can make strategic decisions faster by harnessing the power of data you generate through a digital data backbone.
-
Immunogenicity testing strategies and methodologies are well-established, but we need new approaches to data handling and analysis to streamline reporting.
-
Learn how to build the required data foundations to enable good insights across the BioPharma development lifecycle.
-
Learn how a combination of controlled compliant workflow execution, dramatically streamlined data acquisition and an “audit by exception” approach offers the next phase of innovation within bioanalysis.
-
Find out how you can expedite process understanding in order to enable faster decision making and ensure a view of data for knowledge transfer throughout the BioPharma lifecycle.
-
Design, run, and analyze experiments and processes in a single digital platform for end-to-end context, visibility, traceability, and effective optimization.
-
Check out this discussion on the benefits of microservice architecture and approaches for deploying and managing SaaS solutions utilizing microservice architecture in GxP regulated environments.
-
Learn about best practices for meeting CPV and CPI requirements using purpose-built software that collects and transforms critical data for product lifecycle validation with two technology experts.
-
Discover solutions to create a digital data backbone and best practices for laying the foundation for advanced analytics to make process understanding easier and more efficient.
BROCHURES
- Top 10 Reasons To Move From On-Premise Systems
- Achieving GxP Compliance With Confidence: A Practical Framework
- Efficient Process Lifecycle Management For Optimized Drug Development And Tech Transfer
- IDBS PIMS And Regulatory Compliance
- A Validated Cloud Software Service
- As Biopharma Advances, So Must Our Data Integrity
- Smarter Drug Manufacturing Process Data Management
- A Study Management And Method Execution Platform For Biopharma
- Accelerating Time To Insight Within The BioPharmaceutical Lifecycle
- Tech Transfer And The Need For Digital Transformation
- Regulatory Compliance For Electronic Records And Electronic Signatures
- Power Your Process Development Digital Data Strategy
- Information Security And Business Continuity
- Enabling Data Visibility In Contract Manufacturing Networks
- Data Repository Software To Support Manufacturing Operations
VIDEOS
-
Achieve GxP compliance with confidence. Explore a unified platform that contextualizes all your bioprocessing data, from experiment to product.
-
Are you tired of the inefficiencies and gaps in your current process development methods? Say goodbye to manual documentation and hello to a revolutionary solution designed specifically for your needs.
-
Discover the impact of a cloud-based software platform with practical applications for instrument integration, bioreactor analysis, and more.
-
Learn how Polar, a powerful cloud-based software platform, can optimize your data management, streamline processes, and reduce time to market.
-
Explore a system to help you document unit operations, curate structured and contextualized data in a single platform, perform and analyze DoE studies and drive analytics-powered decision-making.
-
When life sciences manufacturers rely on spreadsheets and paper records, it makes creating, gathering and sharing product and process data burdensome, expensive and prone to data integrity issues.
-
Learn how one leading biotech company achieved adopting new technology to establish a single source of truth across their diverse manufacturing network with compliant, scalable process data management technology.
-
Learn about a data management and collaboration platform that could make your scientists' work days easier.
CONTACT INFORMATION
IDBS UK HQ
Space, 68 Chertsey Road
Woking, GU21 5BJ
UNITED KINGDOM
Phone: +44 1483 595 000
Contact: Sophie Walker
FEATURED ARTICLES
-
A recent survey of over 850 biopharma professionals reveals key challenges and priorities for managing lab data, including scalability and improving research efficiency.
-
Learn how a tiered approach to ADME-Tox studies can help researchers navigate early-stage preclinical research and mitigate the risk of costly failures in drug development.
-
Effectively managing process parameters and quality attributes is vital for successful drug development. Learn how a Quality by Design approach can optimize your processes and ensure product quality.
-
Overcome data integrity challenges in your bioanalytical lab. Learn how a connected data strategy streamlines operations and enhances regulatory compliance.
-
Implementing IDBS’ ELN helped Crown Bioscience realize electronic experimental records, optimizing our business processes and helping them to be more standardized.
-
By leveraging advanced DMPK software solutions, you can streamline your workflows and make informed decisions with confidence.
-
Accelerate drug development with AI. Enhance process intelligence and achieve gold-standard data sets with the right digital tools and improved maturity.
-
Learn about a collaboration that has enabled BioIVT to enhance efficiency and responsiveness to its biopharmaceutical clients.
-
The biopharma industry must shift from data collection to process knowledge, leveraging digital tools to optimize drug development, enhance quality, and streamline tech transfer through digital maturity.
-
Explore strategies for maximizing ROI in pharma through advanced analytics, streamlined operations and an interconnected data management platform.
-
Find out how cloud solutions empower businesses to achieve agility and security in their digital transformation journey, and explore key considerations and insights from real-world success stories.
-
Tech transfer is critical for biopharmaceutical production, but outdated tools can hinder the process. Discover how digital maturity can streamline operations, reduce risks, and accelerate time to market.
-
In the age of digital transformation, AI is no longer optional. Explore how to successfully integrate AI into your organization's operations for a competitive edge.
-
Every domain produces critical data and this data and contexual information should be captured and managed in the most effective and robust manner.
-
Relying on fragmented data becomes problematic when teams need to dissect cell line performance data and that data is not in a digital backbone.
-
The right data management platform can streamline biopharma technology transfer by bridging digital gaps, accelerating commercialization, ensuring compliance, and reducing costs.
-
Why do many BioPharma organizations still struggle to gain the crucial benefits they desire from their data and digital transformation tools?
-
Discover how a comprehensive DoE platform can streamline your drug development process and deliver high-quality results.
-
Explore the story of a global biopharma company's integration of E-WorkBook with their LIMS, automating ADA study reports, saving significant time, and enhancing data accuracy.
-
Discover the significance of digital transformation and its impact on various aspects of organizational operations.