ABOUT ENTEGRIS
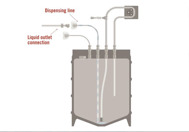
In biopharmaceutical production, each instance where materials or products in development must be stored, handled, or transported presents a risk for breakage, contamination, and expensive product loss. In a modern, decentralized biopharmaceutical production line, materials and products must be kept frozen to ensure product safety, consistency, and shelf life. Because of these new realities, we face more production costs, logistics, speed, and complexity.
Bioproduction today has dramatically increased the value of product in each container. Production processes have been contracted out or may be taking place in different parts of the country or globe. At colder temperatures, containers have considerable limitations; traditional storage bottles are often more brittle at cold temperatures and can break easily, do not pack into containers or store in freezers efficiently and take longer to freeze and thaw due to their shape and volume.
Entegris is in a unique position to help overcome these cold temperature challenges by offering assemblies designed for extreme cold and by partnering with qualified equipment manufacturers specializing in reliable, cold-chain containment. Let us help you gain control over your processes and reduce the potentially enormous financial risk to your valuable products.
VIDEOS
-
Correctly sizing sterilizing-grade filters is crucial for optimizing sterile filtration processes. Learn how Pmax and Vmax methodologies impact efficiency, cost, and filter performance, including the role of pre-filters.
-
Learn practical strategies for optimizing harvest, cell separation, and large-volume freezing to ensure process efficiency, reduce risk, and maintain product viability.
-
Take advantage of our expertise in freeze/thaw. We have tools, expertise, and technologies in one place to enable you to make the right choices in refining your project.
-
Learn how to select the right sterile filtration solution for your specific single or multi-use life sciences applications with Chris Rios, Senior Field Applications Engineer.
-
Discover how can you select the best membrane and filter to meet all the requirements of your life science application.
-
Find out how to select a prefilter for sterile filtration applications and recognize the advantages between various membrane morphologies with Chris Rios, Entegris Senior Field Applications Engineer.
-
Discover sterile filter designs that minimize the amount of product loss that occurs from hold-up-volume during scale-up activities and at each stage of development and production.
-
Watch how our single-use mixing system creates a completely sterile mixing and recirculation process, ensuring your buffer and media are mixed correctly and efficiently.
-
Ross Acucena, applications director, and Donnie Beers, product manager, introduce our single-use mixing system made to be deployed rapidly to help manufacturers meet development, supply, and cost objectives.
-
Ross Acucena, applications director, and Donnie Beers, product manager, introduce our highly functional and adaptable microcarrier cell separation bags designed for single-use processes.
DATASHEETS & BROCHURES
-
Explore essential phosphitylating and phosphorylating agents used in API synthesis and oligonucleotide preparation for cell and gene therapy applications.
-
Discover end-to-end solutions for challenging large-scale oligonucleotide manufacturing. Optimize processes across the entire value chain to enable ultra-high purity and cost-effective commercialization.
-
Learn about a sterile, gamma-irradiated centrifuge assembly that features precision tubing and plugs, as well as detailed tolerances and material specs for reliable integration into workflows.
-
Learn about an innovative microcarrier and cell separation system that streamlines cell processing by delivering sterile performance through an easy-to-use setup that naturally fits into your workflow.
-
Achieve homogenous solutions rapidly with an innovative single-use system designed for sterile, closed mixing of powders and liquids, or liquid-liquid combinations.
-
The Entegris mixing system is designed to maximize simplicity, ease-of-use, and affordability. This single-use bag system creates a completely sterile mixing and recirculation process from start to finish.
-
Learn about the specifications and performance data for UPE vent capsule filters designed with a dual-layer hydrophobic ultra-high molecular weight polyethylene membrane
-
This solution reduces the risk of contamination by aseptically handling samples or aliquots of liquid that would normally have to be collected via open sampling methods such as conical tubes or vials.
-
Improve your handling, efficiency, throughput, and total cost of ownership in bulk-drug substance and cold-chain applications through a reusable, plastic shell solution.
-
This cell banking manifold kit can shorten your seed train process from weeks to days with manifolds that enable biomanufacturers to save time, money, and space while reducing contamination risks during upstream processing.
CONTACT INFORMATION
Entegris, Inc.
129 Concord Road
Billerica, MA 01821
UNITED STATES
Phone: 978 436 6500
Fax: 978 436 6735
FEATURED ARTICLES
-
Phosphorylation is key for bioactives, with phosphoramidites favored for high selectivity. Discover how choosing specific protecting groups and new technologies fine-tunes the synthesis process.
-
Explore single-use 2L face ported bags designed for efficient cell harvest and separation that offer robust film technology, full traceability, and seamless integration with Sorvall BIOS 16 systems.
-
Crucial for efficacy, thermal stability in RNA/DNA therapies hinges on phosphoramidites: A study highlights key factors for process safety and quality in oligonucleotide development.
-
Some membranes attract water ("hydrophilic"), while others repel it ("hydrophobic"). Here, we examine which is best for your application.
-
Explore how this mixing system efficiently handles various powdered media and buffers, ensuring precise solubilization and mixing in applications like cell culture and protein purification.
-
Cell banking stands at the forefront of medical advancement. This guide delves into the steps involved in protecting these priceless resources, from cell collection and processing to storage and cell expansion.
-
An alternative to traditional polyethylene bags is offering cell and gene therapy manufacturers a solution to contamination concerns regarding DNA or RNA fragments.
-
Examine the benefit of using fluoropolymer film bags for the handling and storage of high value oligonucleotides- based payloads.
-
Entegris announces their collaboration with Agilitech, allowing them to offer an extended line of custom, single-use systems for cell and gene therapies.
-
Explore both traditional cold-wall and convection technology options thoroughly to discern which is most suitable for meeting your cold storage requirements for upcoming projects.
WEBINARS
-
This expert panel breaks down the practical realities of today’s supply chain while forecasting how emerging technologies will redefine quality standards.
-
Navigate the complexities of PUPSIT with expert guidance. Discover best practices for assembly design, regulatory compliance, and troubleshooting common challenges in sterile filtration environments.
-
Dive into specific unit operations and case studies that demonstrate how implementing phase appropriate tools can have substantive direct business impacts to cost and development timelines.
-
Unlock insights into sterile gas filtration challenges in bioprocessing. Watch this on-demand session to learn optimization strategies for critical applications and improved filter performance.
-
While AI is an exciting area for the biopharmaceutical sector, other shorter-term and more tangible things can be done to get life-saving medicines to market faster.
-
This presentation emphasizes the significance of minimizing particulate contamination in pharmaceutical and biopharmaceutical products produced through the use of single-use systems.
-
Review data and evidence that demonstrates the benefits of an integrated approach to achieving critical end-user goals such as scalability, efficiency, sustainability, and optimized process economics.
-
Discover how to integrate appropriate materials and tools for oligonucleotide handling, optimize DNA storage using dedicated fluoropolymer film, perform complex biochemical reactions in bags, and more.
-
Explore how filtration manufacturers optimize materials for performance and integration within single-use systems, including the value of optimizing sterile filtration by design and how it integrates and maximizes performance.
-
Learn how utilizing high-density cell banking workflows in combination with your already intensified upstream process can further enhance your process productivity while reducing contamination risks.