Temperature & Containment Controls In ADC Manufacturing

By Matthew Pillar, Editor, Bioprocess Online

Earlier this month, I was hosting a Bioprocess Online Live discussion with leaders from a couple of biotechs with Phase 1 ADC assets in the clinic, when an audience member asked a question about how to address OEB (occupational exposure bands) and containment, and the elevated temperatures needed for ADC production.
Naturally, my panelists – who work for companies that are representative of a large and growing number of ADC-producing biotechs who don’t actually manufacture anything themselves – weren’t quick to answer. The reality of manufacturing in this space is that production of a single ADC asset is dependent on, in most cases, multiple outsourced manufacturing partners. In-the-weeds ADC manufacturing expertise typically lives well outside the four walls of the sponsor companies developing the therapies.
That unique ADC manufacturing management paradigm is fodder for another column. But one of the things I hate most is leaving an audience member’s question unanswered, so I thought it worthwhile to at least attempt to address the OEB and temperature questions here, with an important caveat. I’m not an ADC process development expert (which sounds like a Captain Obvious statement as I type it). What I am is happy to research some (hopefully) helpful resources from people who are experts and offer them here.
Occupational Exposure Bands In ADC Manufacturing
Pharmaceutical manufacturers have long used OEBs to categorize (or, “band”) chemicals based on their adverse health outcomes and potency. Categorizing these chemicals based on established OELs (occupational exposure limits) helps cGMP manufacturers eliminate risk by establishing safe handling guidelines. OELs represent the airborne concentration of a compound to which workers can repeatedly be exposed to during a typical 8-hour day, 40-hour workweek without suffering adverse health effects. In ADC manufacturing, determining OELs requires a hazard assessment to characterize the toxicological and pharmacological properties of the ADC and its parts. These OEL calculations are then used to determine handling and safety measures.
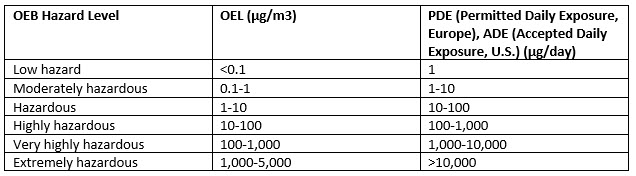
OEBs are then ranked by airborne concentration exposure. Back in 2016, the world-class containment expert Richard Denk offered a peer-reviewed article covering OEBs in ADC manufacturing, which you can access here. That article presented Denk’s contribution of the Containment Pyramid, which identified 6 bands of occupational exposure. Here’s a tabular representation – check out the article above for his original:
Generally, containment measure recommendations correlate with the OEB hazard level:
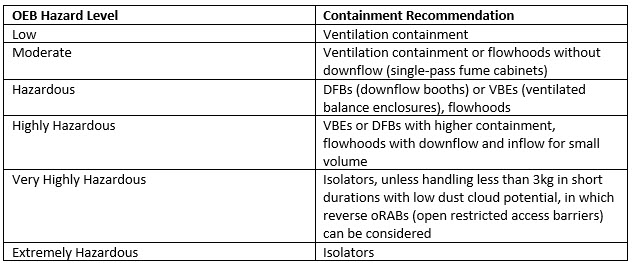
As recently as 2020, Denk conceded that biopharmaceutical agents presenting OELs down to 1 ng/m³ or even below might require the addition of a seventh OEB. Regardless, isolators are the standard in ADC manufacturing, where highly active and extremely hazardous substances like tubulin inhibitors, DNA-damaging agents, and protein toxins, for instance, are common payloads.
ADC Manufacturing Temperature Extremes
Bulk drug substance (BDS) and finished product storage temperature requirements for ADCs are well-documented. Multiple sources call for BDS to be stored frozen at -20°C to -60°C after aseptic dispense in polyethylene terephthalate glycol bottles. Finished products, depending on formulation, call for storage at -20°C to -80°C.
In between those processes, however, the activation and conjugation steps can require multiple elevations and decreases in temperatures (up to 55°C in some patent literature). That calls for containment in sealed, temperature-controlled, typically stainless-steel tanks, with a nitrogen overlay and tight control of pH. Related to the previously covered containment considerations, this step requires precautions including double mechanical seals, positive displacement pumps, and overflow trays to minimize the risk of exposure. Controlled freeze/thaw rates are an important consideration, as slow freezing can result in longer ice crystals that damage antibodies in storage. Repeated freeze-thaw cycles can also cause ADCs to aggregate, which can reduce their effectiveness. Appropriate freeze/thaw rates are dependent on the drug's properties and stability, process constraints, and, of course, cost.
These resources should shed some light on that particular question posed during this month’s Bioprocess Online Live event, and at a minimum, point the asker and the curious in the right direction. I invite readers and guest contributors to please weigh in with their expertise (and, likely, corrections).
I’m certainly no expert, but I’ll be speaking with a couple of folks who decidedly are experts on August 8 at 11 AM ET, and you’re invited to join me. That discussion is on early process development and manufacturing considerations for novel protein therapeutics (specifically, ADCs and fusion proteins), and it will feature SOTIO Head of CMC Meinhard Hasslacher, Ph.D. and Vera Therapeutics SVP of Product Development & Manufacturing Nareej Pakala, Ph.D. We’ll discuss workflow considerations for the race to IND, and the impact of those decisions in late-stage, pre-commercial manufacturing activity. Register for the session (it’s complementary with thanks to Thermo Fisher Scientific) here and bring your questions for the experts.
