Safely Transport ATMPs In Single-Use Bags
In recent years, despite their costs, cell and gene therapies (CGT) have proven to be promising approaches to personalized medicine. One consequence is the need to obtain tissue and/or cellular samples of the patients and transport them to manufacturing facilities for processing and subsequent returning the product to the patient.
Patients needing a cell and gene therapy as an advanced therapy medicinal product (ATMP) rely on their treatment being ready as soon as possible. For example, CAR T-cell therapies are approved for very aggressive and hard-to-treat types of cancer, where time is of the essence.
Cell and gene therapies yield a small volume of individualized product for each single patient. Loss of the shipment or damage to the transported goods have serious consequences for patients.
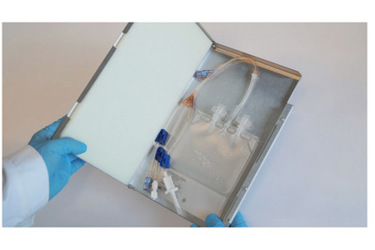
Therefore, reliable, safe, affordable and scalable solutions to these obstacles were needed. RoSS.KSET satisfies all those requirements. It is an integrated solution to protect and safely transport single use bags for small volumes at low-temperature refrigeration.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Bioprocess Online? Subscribe today.
