Melanie 9 Coverage Software
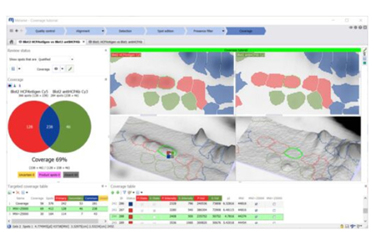
During development and manufacturing of biopharmaceuticals, host cell protein (HCP) impurities must be carefully identified and monitored to guarantee patient safety and drug efficacy. Melanie™ Coverage puts a powerful tool in the hands of scientists developing or utilizing immunoassays such as enzyme-linked immunosorbent assays (ELISA) to monitor HCP impurities in biopharmaceutical products.
The software is focused on the analysis of coverage—the percentage of immunodetection that an antibody reagent offers for the total population of HCPs. It does this by comparing a 2D gel or blot image of proteins detected with an anti-HCP antibody (i.e., secondary image) against an image revealing all HCP antigen proteins (i.e., primary image).
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Bioprocess Online? Subscribe today.
