Balancing Conservative vs. Progressive Approaches To Enable Biomanufacturing Quality By Design
By Jason Dickens and Mike Ponstingl

As the biopharmaceutical industry continues to pursue innovations that streamline processes along the clinical and commercial development pipeline, an increasing number of biopharmaceutical manufacturers have begun to adopt continuous improvement strategies that offer them greater process control, more robust manufacturing processes, and ultimately, higher quality products.
Process Analytical Technologies (PAT), real-time analytical devices and measurement tools, can be used to continuously monitor parts of a process in order to safeguard its quality or identify areas for improvement. PAT are among several tools that can help promote greater process knowledge and enable a Quality by Design (QbD) approach, which in turn helps facilitate improved product quality and mitigate risk.
Designing a control strategy that incorporates PAT requires an understanding of how these technologies can support a sustainable and transferrable manufacturing process. This can be a daunting undertaking, particularly for biopharmaceutical companies that have relied on traditional release testing, but the potential benefits are manifold. Real-time capabilities represent the future of scale up and scale out – companies can stand up new facilities and directly compare their processes from Day 1, enabling a level of control unheard of just decades ago.
Traditionally in the bioprocessing space, the quality of an end product has been determined by post-manufacturing testing. Increasingly, biopharmaceutical companies and manufacturers have begun to incorporate QbD approaches that utilize in-line monitoring to achieve continuous process verification, process control, or real-time release. Real-time release necessitates a more conservative approach to PAT implementation, whereas process control and continuous verification may necessitate a more progressive approach. Determining what approach to take, as well as how to tailor that approach to multiple sites and future business objectives, is critical to a process control strategy that will position a bioprocessing manufacturer for the future.
Developing a Tailored QbD Approach
Biopharmaceutical companies and regulators alike have long advocated for process and monitoring improvements that enhance both the quality and time-to-market of novel and transformative therapeutics. The Food and Drug Administration’s 2004 report, “Pharmaceutical cGMPs for the 21st Century—A Risk-Based Approach,” highlights the importance of early adoption of new technological advances for the industry, including “industry application of modern quality management techniques, including implementation of quality systems approaches, to all aspects of pharmaceutical production and quality assurance.” This focus on quality, coupled with an emphasis on technological advancement, has spurred biomanufacturers to incorporate a QbD approach as part of their manufacturing processes in order to realize measurable gains in quality, timelines, and cost.
Implementing PAT starts with generating a Failure Mode and Effects Analysis (FMEA), a document which details all of the possible failures inherent to a manufacturing process, design, or product. These failures are then prioritized by their potential severity, frequency, and manageability, and addressed systematically to achieve a continuous improvement paradigm within a given process. FMEAs are living documents, updated to reflect new process-level knowledge and product outcomes; an initial FMEA may lead a manufacturer to install process controls at specific points, only to roll back, update, or add to those as data is gathered and analyzed.
There are different levels of rigor that can be applied to a QbD approach, depending on what a manufacturer is trying to achieve through the adoption of PAT. Conservative approaches to PAT that enable real-time release are usually built around off-line analytical techniques, which necessitate a high degree of rigor to ensure that every measurement is achieving a reliable level of selectivity and quantitative capabilities. While online analytics, employed for the purposes of knowledge generation, process control, or fault detection, still require a certain level of rigor, this tends to be less stringent than the level required for offline release assets. The goal of a QbD approach should be first to understand the capabilities of a process; while taking a more stringent and conservative approach can seem like the right move for its focus on real-time release, incorporating less rigorous monitoring techniques can help manufacturers better understand how their process responds to specific variables and seasonal impacts.
Implementing varying levels of rigor to achieve specific ends is a relatively new concept for a marketplace that has been traditionally defined by a singularly conservative regulatory atmosphere. But understanding which PAT approaches can help augment an application’s existing processes, as well as how these can be adapted over time to accommodate growth, can help companies remain competitive in a rapidly changing healthcare landscape.
Employing Customized, Fit-for-Purpose Monitoring Technologies
With more than 40 years of experience across multiple industries, Custom Sensors and Technology (CST) has established itself as a leading provider of robust, fit-for-purpose PAT and accessories. CST has a wealth of experience in designing sensors and monitoring systems that are tailored to a client’s unique specifications, and which allow for seamless integration with existing systems and software. By approaching each application with the goal of providing PAT that achieves the right level of complexity at the right price, CST offers its customers the latitude to target specific improvements, learn more about their processes, and achieve the optimal QbD approach for their goals.
CST’s established technologies and products, which have achieved decades of success supporting the chemical, petrochemical, food, beverage, pulp, and paper industries, have seen commensurate success in the biopharmaceutical space in the last decade. This is due, in part, to the robustness of CST’s equipment and methodologies, including technologies such as absorbance and fluorescence-based photometry, which have proven invaluable across a range of applications. These modalities can also be coupled with other integrated sensors to achieve transferable, replicable, versatile, module and robust monitoring capabilities. These low-cost, easy-to-use methodologies represent a sea-change opportunity in the bioprocessing and pharmaceutical industries, which has experienced a shift in perspective around time-to-market, heralded, in part, by the pandemic response.
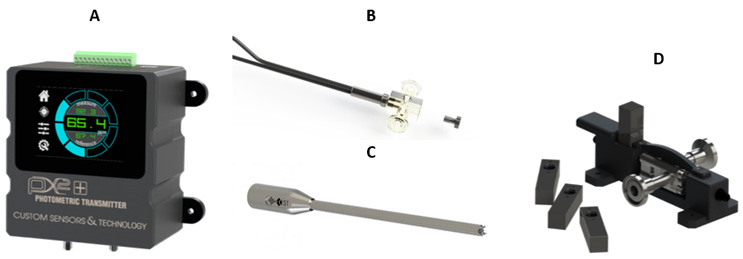
Custom Sensors and Technology’s various PAT solutions include its PX2+ Photometer (A), its single-use fluorescence flow cell (B), insertion Probe (C), and absorbance EZ-CAL flow cell (D).
One of CST’s more popular solutions among bioprocessing manufacturers has been its absorbance and fluorescence photometers and associated flow cells and insertion probes, which are easily integrated in processes such as downstream purification processes. They can also be customized to measure varying concentrations of a range of proteins and other target analytes. As a result of this flexibility and utility, CST saw its sales in biopharmaceutical downstream processing double in comparison to last year. This success is reflective of CST’s commitment to developing and qualifying PAT that are optimized for biopharmaceutical applications.
Ultimately, PAT and QbD approaches represent the next phase in process improvements for biopharmaceutical manufacturers. By working with qualified, experienced technology providers, biopharmaceutical companies can establish a QbD approach that incorporates the right level of rigor to help them understand their processes, achieve continuous improvement, and transfer applications to other sites with ease. With a wealth of experience customizing monitoring technologies for a range of industries, Custom Sensors and Technology can help manufacturers determine the QbD approach best suited to their needs, saving time, money, and resources.
About the Authors
 Mike Ponstingl, president and founder, Custom Sensors and Technology
Mike Ponstingl, president and founder, Custom Sensors and Technology
Mike Ponstingl founded Custom Sensors and Technology in 1980, along with a group of scientists and engineers from Monsanto, and has built the company up into a world-class engineering and process instrumentation company serving global customers. With 41 years of industry experience behind him, Mike has led the company on a journey of strategic growth through his impressive entrepreneurial vision and determination. Mike spent his first 10 year working for Rockwell International, Radian Corporation, and Beckman Instruments before founding Custom Sensors and Technology.
 Jason Dickens, PhD, Technical Consultant
Jason Dickens, PhD, Technical Consultant
Jason Dickens provides technical consultation to Custom Sensors and Technology based upon 20 years of biopharmaceutical process development experience across disparate biotherapeutic modalities and associated biomanufacturing processes. He has also successfully led various advanced process control initiatives within the sector that have enabled innovative GMP process control and real-time release capabilities, as well as enhanced process understanding. His industrial experience includes technical leadership roles at Eastman Chemical Co., GlaxoSmithKline, Biogen, Metabolon, and the Duke Human Vaccine Institute.
