Enabling Advanced Process Control And Continuous Verification For Biopharmaceutical Manufacturing

Quality by Design (QbD) is risk-based amalgam of advanced process controls (APC) that comprise of integrated and aggregated real-time and near real-time (at-line) process analytical technologies (PAT) that enable enhanced process trajectories and intermediate material quality attribute(s) determinations (IMQA). A process control space is derived when this collection of real-time analytics is combined with post manufacture determination of drug substance (DS) or drug product (DP) critical quality attributes (CQAs) or the quality target product profile (QTPP). Furthermore, cultivation of the broader design space via process stretching and failure mode evaluations during process development and scale-up campaigns.1-8 Continuous process verification is an additional QbD beneficial construct derived from a robust APC strategic and enabled design.
The lowest level of quality is dependent upon procedural batch executions and post DS or DP manufacture quality target profile determination where limited or negligible PATs are implemented and utilized within the GMP landscape. A moderate level of quality is comprised of quality target profile determination post manufacture along with the integration of advanced process control (PAT) constructs at critical unit operations or process steps that are prone to failure. The highest level of quality integrates and aggregates PATs throughout the process to mitigate both moderate and high risks. Phase appropriate applicate of APCs as well as commercial scale and demand (e.g., process impact risk assessment) govern the QbD design for a given process platform.
Moderate and high biomanufacturing QbD designs are often associated with high throughput or continuous manufacture process trains. APC through the application of various PATs afford real-time physicochemical attribute determination to derive real-time process control where off-line laboratory analyses are insufficient or impractical. PATs are broadly categorized into two key groups, real-time via integrated sensors and near real-time via at-line analyzers. The former is a key component of the process trajectory ensemble in combination with physical or engineering variables (e.g., pressure, temperature, flow rate, etc.). That is, real-time PATs provide unique temporal quantitative and qualitative capabilities such as real-time concentration of a critical constituent(s), structure as well as heterogeneity or variability information. Furthermore, real-time PATs are either in situ via an in-line probe or flow cell and on-line via slip stream adjacent to a process stream. At-line or near real-time PATs are designed to operate near the manufacturing process or within the manufacturing environment where an in-process acquired sample is analyzed to assess the target material quality attribute(s). These at-line modalities provide an additional mode of advanced process control and often afford either a binary pass or fail result, content value, or a structural indicator. They also follow conventional analytical development, qualification, and validation criteria whereas a real-time PAT often necessitate exhaustive technology selection practices and additional robustness evaluation owing to their routine integrated utilization often deployed across disparate manufacturing environments worldwide.
Commensurate with quality standards, advanced process control and continuous process verification necessitates robust and reliable PATs of both genres described above. Most real-time and several at-line PATs involve spectroscopic analyzers and photometric sensors. Operational and environmental robustness, intuitive operation, readable mechanical and IT integration, across disparate DSs and DPs translation, transferability across manufacturing sites, and capital and sustainable cost of ownership summarize the selection criteria for PATs. While full-spectrum Raman, near-infrared, and UV-vis spectroscopies have emerged as conventional PATs3, 5, 6, they most often require development and sustainment of a complicated multivariate model for each application and product, can be prone to robustness issues, and often require significant capital and cost of ownership. They may also involve movable components, such as variable pathlength technologies.
Photometric PAT sensors, in contrast, are solid-state devices that are far more robust, less prone to environmental factors (temperature and humidity), and only necessitate classic least square calibration or an extinction coefficient when a quantitative result is required. Photometric PATs, thus, are far more sustainable across applications and DSs and DPs relative to full-spectrum spectroscopies. Moreover, photometric sensors are a fraction of the cost, both from capital and cost of ownership perspectives. Photometric sensors include single wavelength, dual wavelength, and multiwavelength configurations. Various well established and robust in-line probes and flow cells are also commercially available with a long history of utilization in industrial applications. Photometric PAT solutions are well suited for broadband spectroscopies such as UV-vis and fluorescence. They are also commonplace for real-time refractive index and turbidity monitoring.
Interestingly, photometric PATs have received modest attention within the biopharmaceutical sector despite their intrinsic robustness benefits.3, 5, 6 Consequently, the chemical industry that increasingly utilizes biomanufacture constructs tends to adopt photometric sensors in lieu of more complicated full-spectrum spectroscopy PATs. Modern chemical plants also share the burden to translate advanced process control capabilities across disparate products analogous to biopharmaceutical manufacture, albeit perhaps to a less degree.
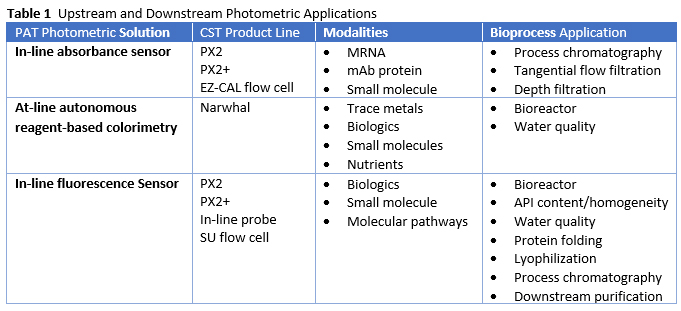
Table 1 summarizes the established applications for robust photometric PATs along with the Custom Sensors and Technology product lines. Most of the applications exist in downstream unit operations, but the upstream opportunities are also attractive and well established in the scientific literature. Integrating aggregated photometric sensors and at-line analyzers across upstream and downstream biomanufacturing unit operations forms a more robust strategic approach as they are more cost-effective and efficient to implement and sustain to enable advanced process control and continuous process verification, to thereby cultivate a desired QbD design as part of an effective quality program.
- Harris, R. J., Determination of critical quality attributes for monoclonal antibodies using quality by design principles. Biologicals 2016, 44 (5), 291-305.
- Bechmann, J.; Rudolph, F.; Gebert, L.; Schaub, J.; Greulich, B.; Dieterle, M.; Bradl, H., Process parameters impacting product quality. BMC Proceedings 2015, 9 (9), O7.
- Biechele, P.; Busse, C.; Solle, D.; Scheper, T.; Reardon, K., Sensor systems for bioprocess monitoring. Engineering in Life Sciences 2015, 15 (5), 469-488.
- FDA Pharmaceutical Quality for the 21st Century: A Risk-Based Approach https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/pharmaceutical-quality-21st-century-risk-based-approach-progress-report.
- Hinz, D. C., Process analytical technologies in the pharmaceutical industry: the FDA’s PAT initiative. Analytical and Bioanalytical Chemistry 2006, 384 (5), 1036-1042.
- Musmann, C.; Joeris, K.; Markert, S.; Solle, D.; Scheper, T., Spectroscopic methods and their applicability for high-throughput characterization of mammalian cell cultures in automated cell culture systems. Engineering in Life Sciences 2016, 16 (5), 405-416.
- Yu, L. X., Pharmaceutical Quality by Design: Product and Process Development, Understanding, and Control. Pharmaceutical Research 2008, 25 (4), 781-791.
- Zobel-Roos, S.; Mouellef, M.; Siemers, C.; Strube, J., Process Analytical Approach towards Quality Controlled Process Automation for the Downstream of Protein Mixtures by Inline Concentration Measurements Based on Ultraviolet/Visible Light (UV/VIS) Spectral Analysis. Antibodies 2017, 6 (4), 24.
