
ANALYTICAL AND QUALITY CONTROL

Analytical Solutions To Help Accelerate Bioprocessing Success
Unlock the power of data-driven bioprocessing. Discover how advanced analytics can optimize your cell culture process, improve performance, and accelerate success.

Validation Of A qPCR Assay For Host Cell DNA Quantitation
Here, we share the development and validation of a new, highly sensitive and accurate integrated solution for detection and quantitation of host cell DNA to help meet regulatory requirements.

BPI Interviews Purification And Pharma Analytics Leader
Jean Luo, VP of Purification and Pharma Analytics, discusses innovations in bioprocessing, including the development of dPCR assays, lentiviral titer systems, chromatography resins, and supply chain.

Rapid Process Development And Technical Support For AAV Scaleup
Accelerate your AAV production journey. Learn how rapid process development and expert support can streamline your path from vial to purified bulk, ensuring scalable and efficient manufacturing.

A Straightforward Path Toward Regulatory Compliance & Data Integrity With Your Microbial Testing Systems
In this webinar, we will share a variety of strategies for implementing and validating microbial identification systems in the cGMP environment, possible difficulties along the way, and a comprehensive solution that addresses these challenges.

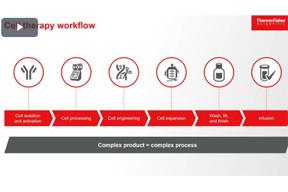
Cell And Gene Therapy Innovations
This interview with a pharmaceutical analytics expert discusses key insights into advances in cell and gene therapy and the strategies used to support customers in improving their therapeutic development.

Digital Solutions For Accelerated Innovation
In this webinar, hear how Applied Biosystems AccuSEQ software serves as an advanced digital tool that can help streamline bioanalytical processes and accelerate the delivery of life-changing therapies.

Key Considerations For Rapid Microbial Methods For Mycoplasma Detection
Learn the importance of demonstrating product specific suitability, defining representative sample, consistent sampling protocols, accommodating volume and turnaround time constraints, and the need to establish equivalence.

Development Of A CHO Medium And Feed System Using Advanced Analytical Tools And Workflow Considerations
Discover how multiomics analysis and optimized formulations in a new cell culture medium deliver higher protein titers and specific productivity for CHO fed-batch systems.

Advancing Vaccine Development With Novel Chromatography Solutions & Quality Testing
Learn more on novel chromatography solutions that can help improve the downstream processes of different vaccine types such as mRNA, recombinant proteins, and Virus-Like Particles (VLPs).

Multi-Omics In Cell Culture Media Design
Discussing the evolution of medium design for cell culture bioprocessing, and the role that multi-omics and bioinformatics can play in improved medium development.

Quality Roundtable: Raw Material Planning For Tech Transfer And Scaling Biologics
Join this roundtable of biopharmaceutical professionals as they share their insights to help you navigate important quality and planning decisions for the progression of your biologic.
Maximize Quality Assurance Through Rapid Sterility Testing
This webinar explores mitigating the risk of product loss, and production delays, as well as the critical role rapid sterility testing plays in ensuring the quality and safety of cell therapy products.

Dry Powder Media Manufacturing Grand Island Expansion
Take a video tour of our newly expanded dry powder media manufacturing facility in Grand Island, New York. The expansion has added more than 45,000 square feet of Animal Origin Free (AOF) manufacturing space to help meet increasing global demand.

Key Attributes To Monitor During Cell Line Development Of Complex Proteins
In this segment of the Bioprocess Online Live event Early Process Considerations For Novel Protein Therapeutics, panelists discuss key attributes to monitor during cell line development processes.

Innovations In Mycoplasma And Sterility Testing For Biopharma
In this webinar, our expert speakers will discuss the types of analytical testing that can be effectively employed in the early stages of therapeutic development and subsequently scaled to meet production challenges.

Simple In-House Mycoplasma Testing Method For Regulatory Expectations And Rapid, Confident, And Actionable Results
In this webinar, we present an overview of worldwide regulatory guidance for mycoplasma testing, as well as a mycoplasma testing method that has been accepted by regulatory authorities worldwide across various therapeutic modalities.

Explore The Analytics Knowledge Hub
Discover smarter solutions with the new Analytics Knowledge Hub that provides articles, webinars, e-books, and infographics designed to enhance and streamline your bioprocess workflow.

Next-Gen Disease Models And Cell Therapeutics: Innovations In AI, Omics And Collaboration
Learn how to leverage AI, omics integration, and collaborative research to build more physiologically relevant disease models and accelerate the development of next-gen cell therapies.

A Successful Journey To Cell Therapy Manufacturing
Learn about the three main areas to consider when you're preparing to commercialize a cell therapy, including starting/raw material, the controlled manufacturing process, as well as testing safety and quality.
Leveraging Rapid Sterility Testing To Advance Cell Therapy Production
Explore the crucial role of rapid sterility testing in cell therapy manufacturing. This presentation delves into the benefits of swift, accurate detection for product quality and patient safety.

Cell Culture Media Manufacturing, Paisley, Scotland
Learn more about our cell culture media manufacturing global network, including details of the Paisley, Scotland site's capacity expansion supporting the bioprocessing industry's rapidly growing needs.

Innovative Strategies For Residual DNA And Viral Titre Quantitation
Discover how digital PCR improves the accuracy of residual host cell DNA and viral titre quantification in biotherapeutic manufacturing to enhance product quality, safety, and regulatory compliance.

Successful Microbial Testing And Identification
In this presentation, one of our industry-leading customers discusses how they've successfully implemented the Applied Biosystems MicroSEQ Microbial Identification System in their laboratory testing.

Interview With Nico Chow And Sandi True, Field Applications Specialists
Hear Nico Chow and Sandi True, Field Applications Specialists, discuss working with customers to help them evaluate and implement Residual DNA Quantitation, Mycoplasma Detection, and Microbial Identification Assays.

A Straightforward Path Toward Regulatory Compliance, Data Integrity, And Computer Systems Validation
Learn practical strategies for validating microbial identification systems in cGMP. Understand regulatory guidance and master a complete Computer Systems Validation plan for full compliance.

Interview With Pharma Analytics Field Application Specialist - Sandi True
Hear Sandi True, a Field Applications Specialist discuss helping customers navigate challenges often encountered when evaluating and implementing Residual DNA Quantitation, Mycoplasma Detection, and Microbial Identification Assays.

Multi-Omics And Bioinformatics In Cell Culture Media Design
Learn about the impact of a multi-omics analysis, utilizing proteomics and metabolomics, in the development and optimization of media with the goal of improving titer, and making development and manufacturing more efficient.
Implementing Rapid Microbial Identification In Biotherapy Manufacutring
Learn about rapid microbial identification strategies that enhance environmental monitoring and compliance with regulatory requirements for your manufacturing processes.

Quality Roundtable: Optimizing Biologics Manufacturing Processes With Raw Materials
Improve your commercial manufacturing process with advice from regulatory and raw materials experts on the development journey from pre-clinical to licensure.
