ABOUT ABZENA
Abzena is the leading end-to-end bioconjugate and complex biologics CDMO + CRO. From discovery through commercial launch, we support customers with fully integrated programs or individual services designed to de-risk and streamline the development of new treatments for patients in need. With the ability to tailor our strategy and customer experience to each project, Abzena develops and implements innovative solutions that enable biotech and biopharma companies to realize the full potential of their molecule. Our global locations include San Diego (CA, USA), Bristol (PA, USA), and Cambridge (UK).
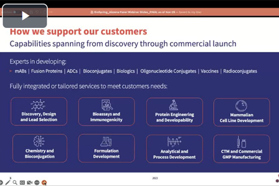
Experts in developing:
Antibodies, Antibody Fragments (Fabs), Antibody Drug Conjugates (ADCs), Bioconjugates (peptides, oligonucleotides, immune-modulators), Bispecific Antibodies, Monoclonal Antibodies (mAbs), Radioconjugates, Fusion Proteins, Growth Factors, Recombinant & Conjugate Vaccines, Antibody & ADC Biosimilars.
Capabilities spanning from discovery through commercial launch:
- Discovery, Design and Lead Candidate Selection
- Comprehensive Analytics, Bioassays and Immunogenicity
- Protein Engineering and Developability
- Antibody Design and Development
- Mammalian Cell Line Development
- Linker Payload Design and Synthesis
- Analytical Method & Formulation Development
- Process Development and cGMP Manufacturing
- Technology Transfer & Scale-Up
- Regulatory Support
Propriety solutions for improving development
EpiScreen®- a robust platform which assesses immunogenicity via flow cytometry and completely avoids radioactive assays, delivering better, more granular candidate selection data.
Composite Human Antibody™ – a deimmunization platform used for designing safer antibodies which maximizes the full potential of the drug.
Composite Proteins™ - a deimmunization technology that designs safer therapeutic proteins, devoid of human T cell epitopes, to minimize potential immunogenicity in patients without compromising activity.
ThioBridge™- a next generation conjugation technology proven to enhance ADC development by overcoming issues with existing technologies to improve stability, potency, and efficacy.
LabZient ™– our analytical platform that combines predictive in-silico evaluation with laboratory methods to de-risk the application of platform analytical procedures and expedites the pathway to IND.
Connect with us today at info@abzena.com or by visiting Abzena.com to discuss your biologic or bioconjugate drug program’s needs with our experts.
ON-DEMAND WEBINARS
FEATURED BROCHURES
CONTACT INFORMATION
Abzena
6325 Lusk Blvd.
San Diego, CA 92121
UNITED STATES
Phone: 858-550-4094
Contact: Kimberly Burrell
ABZENA’S GLOBAL OPERATIONS
San Diego, CA, USA
2858 Loker Ave E. Suite 100
Carlsbad,
CA 92010
USA
+1 858-550-4094
San Diego, CA, USA
11425 Sorrento Valley Rd.
San Diego,
CA 92121
USA
+1 858-550-4094
Bristol, PA, USA
360 George Patterson Blvd.
Bristol
PA,19007
USA
+1 215-788-3603
Cambridge, UK
Babraham Research Campus,
Cambridge,
CB22 3AT
United Kingdom
+44 1223 903498
FEATURED NEWS
- Abzena And Mabqi Announce Strategic Partnership To Offer Integrated Discovery Through Development Solution
- Abzena Enhances AbZelectPRO™ Cell Line Offering With New GS Knockout Platforms
- Abzena Announces Establishment Of Scientific Advisory Board To Support Innovation Strategy
- Abzena Strengthens Board With Appointment Of Biopharma Industry Leader, Dr. Moncef Slaoui
- Abzena Expands Analytical Capabilities To Include GMP Cell-Based Potency Testing Across US & UK Sites
- Abzena Announces Major Expansion In Microbiology Laboratory Space In Support Of Biologic Manufacturing
- Abzena Launches EpiScreen® 2.0 Immunogenicity Platform
- Analytical Platform LabZient™, Expedites The Path To IND For Antibodies