Using A CMO To Streamline Process Characterization
By Evan Pasenello and Daniel Sayut
While traditional small molecule drug products usually consist of pure chemical substances that are easily analyzed after manufacture, biologics such as monoclonal antibodies (mAbs) are much more complex. Not only does this make their production considerably more challenging, but it also means that identification of clinically active components is extremely difficult. Because of this, biologics are instead defined by their manufacturing process, which requires complete understanding through formal process characterization.1.
Typically carried out during the late stages of drug development, following phase II clinical trials, process characterization is an essential component of process validation. Providing an in-depth understanding of a drug product to establish effective process control strategies, process characterization helps to define the critical product and process parameters and determine the proven acceptable ranges for efficient manufacturing. Without thorough and comprehensive testing data a biologics license application (BLA) for the manufacture of a biopharmaceutical for commercial distribution will invariably be denied by regulatory bodies such as the US FDA.
Even a small change to the structure of a biologic during the manufacturing process can result in a significant impact on safety, efficacy and function. Something as simple as a deviation in pH, a temperature fluctuation, a variation in the method of thawing a vial or the switch to a different consumable supplier can affect a biopharmaceutical’s identity, purity or potency. By knowing in advance the influence of different manufacturing events on a biologic and being able to provide supporting data to back up these occurrences through process characterization, the risk of manufacturing failures and regulatory delays is significantly reduced.
Process characterization involves the determination of acceptable ranges for many hundreds of parameters to ensure manufacturing consistency by providing a thorough understanding of the process. One approach to the analysis of process characterization data is statistical in nature, yet this requires a huge amount of work and can dramatically extend timelines if the necessary expertise and equipment are not available in-house. Risk-based and failure-mode approaches are also feasible however the former can be limiting since it treats each possible source of failure in isolation, while the latter may be flawed if a potential failure mode is omitted. Since such a huge volume of data is generated, process characterization should ideally begin at least 1-2 years before a BLA is filed.
By establishing a platform approach to process characterization, which has supported the successful approval of several marketed biologics, scientists at the AbbVie BioResearch Center (ABC) ensure the entire process is robust, right first time, and results in a highly consistent product. AbbVie’s five-step process results in a thorough understanding of the biologic and process control strategies to ensure drug safety, purity, and potency at the commercial scale, and since it is much harder to implement changes post- commercialization, continued process validation is employed.
Step 1: Draft a Master Plan
A detailed master plan describes the manufacture and evaluation of the biologic from start to finish, including strategy and rationale for approaches to small-scale characterization and full-scale validation. Also defining the efforts which will be made to implement and complete the various activities according to current Good Manufacturing Practices (cGMP), the master plan ensures a well thought-out, justified and complete process characterization.
Step 2: Perform Risk Assessments
Risk assessments are performed on process characteristics and product quality attributes to generate a prioritized list of parameters to be evaluated during process characterization. Duplication of work can be prevented by referring to risk assessments carried out for a previous process characterization project.
Step 3: Characterize the Cell Culture and Purification Processes
mAbs are typically produced by cultured cells in bioreactors, from which they are subsequently harvested and purified. Characterization of these processes is broken down into five activities:
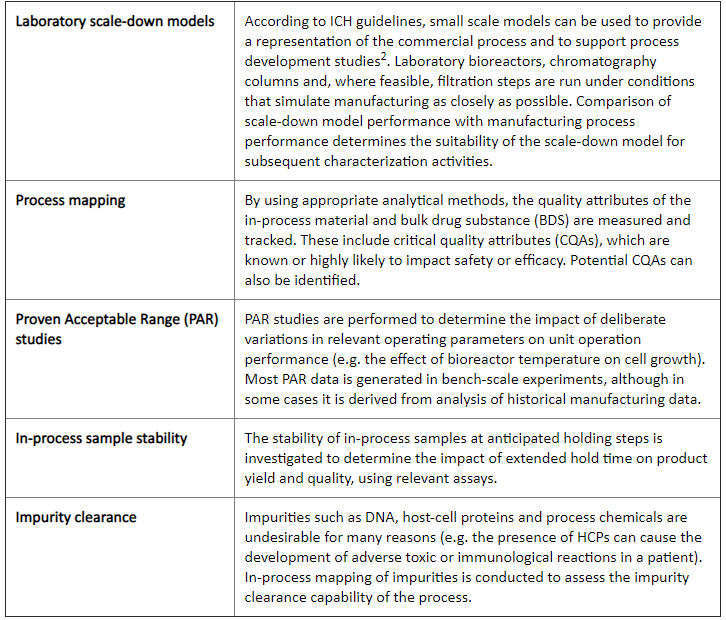
Step 4: Post-Characterization Risk Assessment
A second round of risk assessments is performed on the process parameters, based on the data generated from process characterization studies. The outcome is a listing of critical and non-critical parameters, as well as a ranking of the critical process parameters.
Step 5: Process Justification Report
Summarizing the overall control strategy for manufacturing the biologic, this report provides justification of the proven acceptable ranges for manufacturing operating parameters. It also represents a basis for process validation. As well as using process characterization data, the process justification report draws on historical manufacturing data.
CMOs as process characterization partners
While some major pharmaceutical companies have the expertise, resources, and facilities to handle process characterization, some stakeholders involved in clinical trials of a biologic are wise to partner with an experienced CMO to handle this important stage of a drug’s lifecycle. This can reduce the cost and time of process characterization, resulting in faster progression to regulatory approval and market.
Experienced CMOs offer many advantages, not least that having performed process characterization studies previously they can offer a well-established platform for planning, assessment, analytical studies, data analysis, and review to support market approval. Additionally a CMO will have in place the necessary, and often expensive, equipment required for biopharmaceutical testing, accompanying this with a pool of highly experienced personnel.
Access to specialized instrumentation, administered by experts, can streamline process characterization by preventing the need to out-source to different service providers and can also substantially reduce testing time. AbbVie offers wide-ranging expertise in scale-down models, high throughput systems and experimental design.
Thorough process characterization provides a full understanding of the biopharmaceutical and the processes under which it can be efficiently, compliantly manufactured for safety, purity, and potency. This benefits the biologics manufacturer, the regulatory agencies and ultimately the patient. In contrast, process characterization that has not been performed correctly can lead to issues with regulatory compliance or manufacturing performance. These can force costly re-work and substantial delays in getting to market or can result in tight tolerances for the proven acceptable ranges, greatly restricting manufacturing.
Contact us to talk about process characterization for your biologic drug. As a division of AbbVie, a leading biopharmaceutical company, AbbVie Contract Manufacturing (www.abbviecontractmfg.com) has carried out process characterization for many the biopharmaceuticals within our own pipeline, as well as performing over a dozen process characterization projects for third parties. We also offer wide-ranging expertise in biologics manufacturing, cold chain, and packaging.
