Unlocking The Pharmacological Potential Of Antibodies

By Tyler Menichiello, Chief Editor, Bioprocess Online
Therapeutic antibodies are one of the pioneering modalities in the biopharmaceutical industry, consistently occupying spots on the top-selling drugs list in recent years (e.g., AbbVie’s Humira held the top spot for six years straight). Despite being one of the oldest and most established modalities, mAbs are still improving and taking on new forms — from ADCs to increasingly complex multispecifics. The way companies are discovering these molecules is also evolving.
Conventional mAb discovery relies on high-throughput screening to identify molecules with the highest binding affinity to targets of interest. However, as Abalone Bio’s CEO and co-founder, Richard Yu, Ph.D., explains, screening based on affinity interactions doesn’t always reveal the most therapeutically relevant antibody. Abalone was born out of one simple idea: What if you could screen for the most pharmacologically active mAbs?

Confidently Targeting GPCRs With FAST
Perhaps the strongest sign of Abalone’s confidence in its platform is the decision to target GPCRs — one of the most notoriously difficult target classes for therapeutic mAbs. GPCRs are a challenging target class because of their limited and highly variable extracellular epitopes. For context, only nine GPCR targets have ever been successfully activated by antibodies, and Abalone is responsible for two of them, according to Yu.
“We’re hot on the heels of getting some data on a couple more,” he tells me. He seems confident in the company’s ability to scale the platform and apply it to other targets. “Of course, biology being what it is, there may be some problematic [targets], but thus far, it’s been working well for us.”
Abalone’s pipeline spans three major buckets: metabolism, inflammation, and oncology; all of which contain GPCR targets. Its lead program is a CB2-targeting antibody in preclinical development for liver fibrosis.
While other companies may be working to find GPCR inhibitors, Abalone differentiates itself by focusing on GPCR targets that require activation.
Engineering The FAST Platform
This philosophy around differentiation extends to the company’s use of AI as well. “To say you’re using AI in your company right now, it’s almost like saying, I use PCR in my company,” Yu says. It’s not differentiating unless you’re inventing a novel, next-generation PCR method. Similarly, any company with an AI-based platform that wants to stand out should be focused on generating unique data sets, according to Yu. That’s exactly what Abalone is planning to do with its FAST platform, which uses yeast cells to individually test antibody variants. The name Abalone is actually a nod to the platform, Yu tells me; “Every cell tests one antibody alone.”
It starts with the company’s FAST yeast cells. These cells are engineered to express human GPCR proteins, as well as millions of different antibody variants —with each cell producing its own unique variant. These antibody variants stay restricted to the cells they come from, Yu explains, and when a variant correctly binds to its respective cell’s GPCR, it triggers a signaling cascade that promotes cell growth and proliferation.
“It basically is poking itself to see what it does,” Yu says. By design, the yeast cells with optimal variants outcompete the others in a Darwinian, “animals on the Serengeti situation,” relying on nature to reveal the best antibody design. The company then uses AI and ML to mine the growth curves for the most promising candidates. The added benefit is that this growth-based selection automatically excludes poorly expressed antibodies.
Why Focus On Activating Antibodies?
“There are a lot of drug targets that, if we could more specifically activate or increase the activity of this [target], it would be a great way to treat this disease,” Yu says. This idea addresses a gap that exists in therapeutic mAbs: The gap between molecules that act simply as “bouncers” or inhibitors in the body and molecules that instead activate or modulate targets to produce a pharmacological benefit.
With this goal in mind, it’s not enough to just screen for affinity, Yu tells me. “Binding of course is important,” he says, “but it’s not tantamount to function.”
Just because an antibody binds tightly to the target, doesn’t necessarily mean it’s bound in the right place. He uses a scissor analogy to explain this concept (illustrated below). You can hold scissors tightly by the blades, which symbolizes a high-affinity interaction, but that doesn’t help you cut anything. However, if you hold them loosely and in just the right places, you can cut away to your heart’s content.
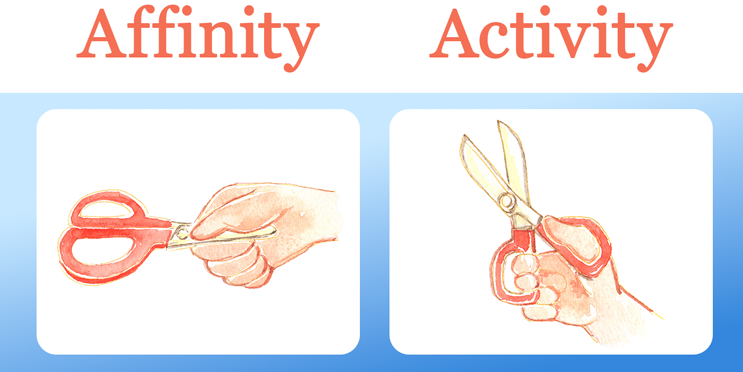
Yu believes designing antibodies that bind in just the right way to activate or modulate a targeted pathway will open up a new avenue of therapeutic potential for mAbs. If you’re interested in learning more about Abalone’s FAST platform, check out my full conversation with Dr. Yu on Better Biopharma, the official podcast of Bioprocess Online. Stay tuned for new episodes, released every other Wednesday, to learn what leaders across biopharma are doing to move the industry forward.
