Tracking 4 Payload Trends In Drug Delivery Development
By Alexander K. Aust and Abigael Frederick, Aust Business Solutions
Lipid nanoparticles (LNPs), polymer nanoparticles (PNPs), and other drug carriers exhibit the capacity to transport a diverse range of payloads, encompassing drugs, nucleic acid therapeutics, vaccinations, functional agents, combinations of compounds, proteins, peptides, and beyond.
Alongside the continuous growth in carrier research and development, market attention has also shifted toward broadening the spectrum of available payloads and enhancing compatibility. This dual focus on expanding both carrier capabilities and payload versatility underscores the dynamic evolution of these delivery systems, reflecting their potential to cater to a wide array of therapeutic applications.
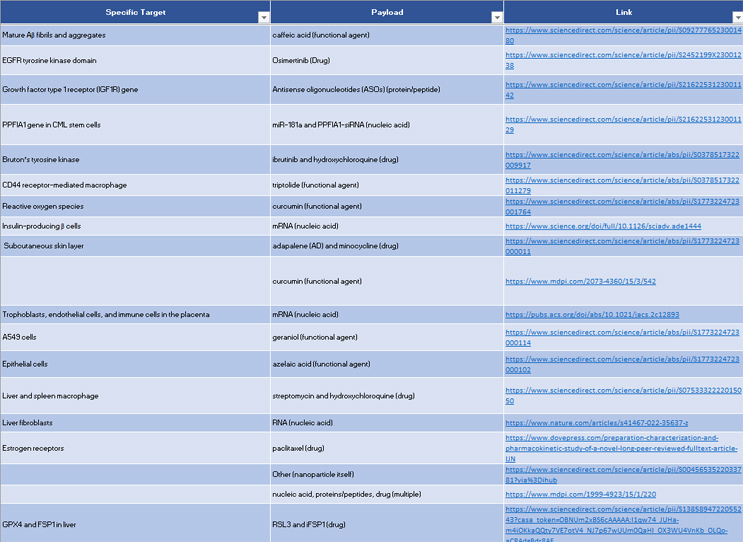
Here are four payload research trends we tracked in 2023. The database below updates our first report and database on LNP, PNP, and other carrier research trends with the latest research plus details about payloads, which were not included in the first database.
Drug Payload
The most popular payload for LNP and PNP technologies is drugs. These compounds range from chemotherapy and tumor treatments to antibiotics and pain relief therapeutics. Almost half of the carrier technology is geared toward encapsulating commercial therapeutics to reduce drug biodegradation and more precisely target tissues of interest to decrease off-target side effects.
Nucleic Acid Payload
With the rising popularity of mRNA vaccinations, there is a noticeable shift in LNP and PNP encapsulation efforts toward nucleic acid payloads. This trend, however, extends beyond mRNA alone. Emerging technologies, including plasmid DNA and CRISPR-Cas-9 systems, are now integral components of innovative carrier designs.
The emphasis lies in harnessing these diverse payloads for applications in gene editing and disease treatment. As nucleic acid therapeutics represent a relatively new frontier, the exploration and incorporation of such payloads are expected to drive ongoing advancements in LNP and PNP engineering. This evolving landscape holds promise for the continued evolution of these delivery systems, opening avenues for novel and sophisticated approaches in genetic medicine.
Protein/Peptide Payload
Protein and peptide therapeutics have emerged as promising modalities for treating various diseases, owing to their specificity and ability to target specific molecular pathways. However, their clinical translation faces challenges related to stability, delivery, and immunogenicity.
LNPs and PNPs provide a protective and controlled environment for proteins and peptides, shielding them from enzymatic degradation and promoting sustained release. The LNP and PNP delivery systems enhance the bioavailability of these therapeutics, enabling effective transport across biological barriers. This approach not only improves the pharmacokinetics of protein and peptide drugs but also minimizes potential immunogenic responses.
Functional Agent Payload
LNP and PNP technology extends beyond conventional commercialized and patented drugs, embracing a broader spectrum that encompasses herbal remedies and experimental therapeutics. This expansion has proven particularly valuable in addressing challenges related to drug resistance and adverse side effects. While herbal remedies and experimental therapeutics may be inherently unstable under biological conditions, the application of LNP and PNP technology has effectively enhanced their stability and longevity as therapeutic agents.
Notable examples of such compounds include coenzyme Q10, caffeine, and safflower extract, among others. These encapsulated compounds exhibit the potential to target diverse diseases and conditions, ranging from cancer and neurodegenerative diseases to diabetes. The versatility of LNP and PNP technology in encapsulating a wide array of therapeutic agents opens new avenues for the development of treatments with improved efficacy and safety profiles.
About The Project
This series tracks new research on lipid and polymer nanoparticles, along with new potential carrier technologies.
After COVID-19, pharmaceutical companies directed their attention to messenger RNA (mRNA) vaccine manufacturing. But nucleic acids are notoriously unstable, and vaccine manufacturers gravitated toward lipid and polymer system formulations to encapsulate these therapeutics. A wave of new research began to gather.
We saw new research pouring out of corporate and university labs on the heels of advancements in nucleic acid therapeutic capabilities, including specialized medicine, cancer treatment, gene therapy, and vaccine development.
We began cataloging new research on PNP, LNP, and other carriers as it was published. Over time, we saw trends emerge, most recently with a heavy focus on oncology, neurology, and infectious disease therapies, as well as mRNA. We realized many drug developers working on mRNA and other modalities could benefit from a public database of LNP and PNP research as they search for ideal couriers to deliver their payloads.
How To Use The Data
Our data provides a far-reaching review of the LNP, PNP, and other drug carrier research landscape from 2020 to 2023. We hope that drug developers find the database useful when researching formulations for specific indications and targets.
Researchers can filter the records to find journal articles related to specific target tissues and, in many cases, target cells. They can sort based on the lipid or formulation studied. In addition to carrier formulations, researchers can also sort the database to find journal articles related to specific payloads.
Each record includes the journal title, the year published, and a permalink to the publication. Contact the authors with questions at alex@austbs.com.
About the Authors:

Alexander Aust, M.S., MBA, is the cofounder and owner of Aust Business Solutions, a pharmaceutical consultancy based in Maryville, TN, where he focuses on strategic planning and partnerships and technical support. The firm provides CMC and project management services, assists with regulatory submissions, and helps to implement new technology. Aust serves as a senior project manager for the Parenteral Drug Association. He earned his MBA from Ball State University and an M.S. degree from Purdue University in molecular and cellular biology.

Abigael Frederick is a business analyst intern with Aust Business Solutions. She earned her microbiology B.S. from Indiana University along with a minor in chemistry. She is beginning her Ph.D. in biochemistry, cell, and developmental biology at Emory University. She spent her college career exploring pharmaceutical and academic research with a focus on immunotherapy, bacterial genetics, and drug delivery systems