The mRNA Express Kit Supports Quantitative, Competitive RT-PCR
By Shiliang Qin and Michael Brush, RNAture
Competitive reverse transcription PCR (RT-PCR) has become a useful tool for quantifying the expression levels of numerous genes. Here, RT-PCR is used to quantify the expression levels of human b-actin in HS587T cells using messenger RNA isolated with the mRNA Express Kit from RNAture.
Methods
Competitive RT-PCR was performed following the recommendations of Freeman, et. al.1 Primers were chosen to amplify a 420 base pair fragment of the human b-actin gene. A homologous, synthetic b-actin mRNA competitor was constructed with a 50 base-pair deletion. After reverse transcription of this competitor, amplification using the wild-type primers produced a 370 bp fragment.
Messenger RNA was isolated from HS587T human breast cancer cells with the mRNA Express Kit. To begin, 4500 HS587T cells were applied to each of six wells on a RiboCap Filter Plate. The cells were washed with PBS by centrifugation. 50 µl of Lysis Buffer containing between 1.3 x 106 and 3.3 x 108 competitor molecules were applied to the appropriate wells and incubated at room temperature for 5 minutes. The lysate was transferred to the GenePlate by centrifugation (670 x g for 5 minutes) and allowed to hybridize for 15 minutes at room temperature. Following three washes with Wash Buffer, a two-step RT-PCR reaction was performed.
The reverse transcription reaction was conducted at 42°C for 50 minutes in 20 µl reaction volumes using 100 units of M-MLV reverse transcriptase per reaction. The wells were washed twice with 10 mM Tris, pH 7.5, then subjected to 25 cycles of amplification using a single set of b-actin specific primers in 20 µl reaction volumes.
The PCR products were analyzed by electrophoresis through a 2% agarose gel pre-stained with ethidium bromide. At the completion of electrophoresis, the gel was photographed with Polaroid Black and White Film Type 665 under UV light. Scanning densitometry was performed using a Personal Densitometer SI from Molecular Dynamics. The ratios of the densities of the competitor and wild type b-actin bands were determined. The logs of these values were plotted against the logs of the number of b-actin competitor molecules originally applied to the wells on the RiboCap Plate.
Results
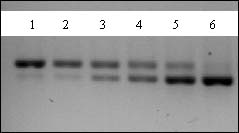
Figure 1. Analysis of RT-PCR reactions. Image of an agarose gel showing the results of the RT-PCR of b-actin mRNA isolated from HS587T cells. The two bands visible are the 420 bp wild type b-actin band (top) and the 370 bp homologous deletion mutant competitor band (bottom). Lane 1: 1.3 x 106 competitor molecules; Lane 2: 4.0 x 106; Lane 3: 1.2 x 107; Lane 4: 3.7 x 107; Lane 5: 1.1 x 108; Lane 6: 3.3 x 108.
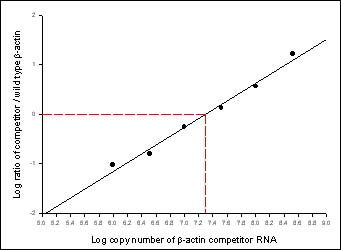
Figure 2. Analysis of competitive, quantitative RT-PCR. The densities of the bands in Figure 1 were determined, and the log of the ratio of the competitor to wild type bands was calculated for each reaction. These ratios were plotted against the log of the number of competitor molecules. The total number of b-actin copies in the HS587T cells applied was determined at the point where the band ratios are equal (log = 0). Here, 4500 cells contain 1 x 107.3 (19.95 million) copies, or about 4400 copies of b-actin mRNA per cell.
Conclusions
Messenger RNA isolated by the mRNA Express Kit was used successfully with a competitive, quantitative RT-PCR methodology to quantitate the expression levels of human b-actin in HS587T cells. About 4400 copies of b-actin occur in each HS587T cell under the culture conditions employed for this example. This number falls within the reported range of message copies expected for an abundant message in mammalian cells.2
References
- Freeman, W.M., S. J. Walker, and K.E. Vrana. 1999. Quantitative RT-PCR: Pitfalls and Potential. Biotechniques 26:112-115.
- Alberts, B., D. Bray, J. Lewis, M. Raff, K. Roberts, and J.D. Watson. 1989. Molecular Biology of the Cell (2 ed.), pp. 528. New York: Garland Publishing.
For more information: RNAture Inc., 1003 Health Sciences Rd. West, Irvine, CA 92612-3054. Tel: 877-327-8762. Fax: 949-725-2788. Email: info@RNAture.com.
