The Latest Techniques And Technology For Differentiating Capsids
Adeno-associated viruses (AAVs) consist of a capsid composed of proteins and a cargo of single-stranded DNA. Researchers are continuously modifying the capsid and cargo to optimize transduction and expression.
Ever since AAV became a choice of vectors for gene therapy, the most talked about quality attributes have been the percent empty and percent full capsids. The process of packaging DNA into capsids during biologic production is indeed complex and can vary based on the multiple factors. Scientists and regulatory bodies have always wondered about the following questions:
- What proportion of the total capsids made are empty and full?
- Are empty capsids completely empty?
- Can there be an interruption in cargo packing?
Numerous bioanalytical companies have innovated technologies and methodologies to address these exact questions. In this article, I will go over the techniques available in the market, the merits and drawbacks of each method, and the key questions to answer before selecting a method of choice for empty/full analysis.
Various biophysical properties of empty and full capsids like surface charge and mass were utilized to develop a method for assessing quality attributes. The gold standard method for decades has been sedimentation velocity analytical ultracentrifugation (SV-AUC), in which the sample is subjected to high centrifugal force and the separation/sedimentation is based on the molecular weight of the particle. The movement is monitored using an optical system and the sedimentation coefficient is determined. The measurements are accurate; however, the challenges with this technique are the large sample volume required, a shortage of skilled technicians, and low throughput.
Anion exchange (AEX) chromatography separates particles on the basis of charge. The charge of full capsids differs from that of empty capsids because of the negative charge of the genome DNA. The negatively charged molecules are retained on the column, while positively charged or neutral molecules elute earlier. This is achieved by gradually changing the ionic strength of the mobile phase. The method is extremely sensitive to the changes in pH and ionic strength, which makes it difficult to transfer.
Mass photometry (MP), a newer technology developed by Refeyn, measures the molecular mass of individual particles based on the light they scatter as they pass through a laser beam. Major challenges include interference from the sample matrix and external stimuli like vibration affecting scattered light.
Another commonly used method for determining percent full is to calculate the ratio of total cargo concentration to total protein concentration. Cargo content is typically measured using PCR-based assays such as droplet digital PCR (ddPCR), quantitative PCR (qPCR), or digital PCR (dPCR), while intact capsid concentration is measured using an enzyme-linked immunosorbent assay (ELISA)
Here are some of the leading products:
- LabChip by Revvity offers a kit capable of determining the cargo and capsid concentration by using a microfluidic capillary electrophoresis method.
- Bio-Rad’s Vericheck Empty-Full Capsid kits leverage the ddPCR method to quantify capsid concentration by using antibodies conjugated with oligonucleotide linkers and, on ligation, the nucleotide fragment is measured to obtain total capsid concentration while cargo concentration is measured simultaneously.
- Tessie by Nanomosaic uses nanoneedle sensors coated with hundreds of antibodies specific to the capsid and the cargo modified during sample processing.
In certain methodologies, the percent empty/full is either derived from the results of two entirely separate assays or assessed using a single integrated assay. This can lead to significant variability, making comparisons to orthogonal methods less precise. Furthermore, many of these techniques operate under the assumption that each capsid contains only a single cargo, which may not always be accurate. Nonetheless, the effectiveness of these methods should not be underestimated, as they are capable of simultaneously evaluating three to five CQAs.
Other Methods
Some methods for empty/full analysis use input values like extinction coefficients or full-length cargo size to inform percent full.
Stunner by Unchained Labs is one such technology that uses UV/vis concentration, dynamic light scattering (DLS), and static light scattering (SLS) for percent measurement.
Multi-angle light scattering (MALS), when paired with size exclusion chromatography (SEC), separates complex AAV mixtures based on size. When MALS is paired with asymmetric flow field-flow fractionation (AF4), it employs laminar flow combined with a perpendicular crossflow field to separate molecules based on their size. This method is gentler on a broader range of samples but is more complex to run.
The MALS detector measures the intensity of light scattered at multiple angles by AAV particles, providing absolute molecular weight and size (radius of gyration). This enables precise characterization of AAV capsids, including percent empty/full.
Imaging methods like cryo-EM are also capable of measuring percent full. Cryo-EM allows visualization of AAV particles at high resolution. It distinguishes between full, partially filled, and empty capsids based on electron density differences within the particles. These methods may serve as effective qualitative visualization tools; however, their application for quantitative assessments is not consistently dependable.
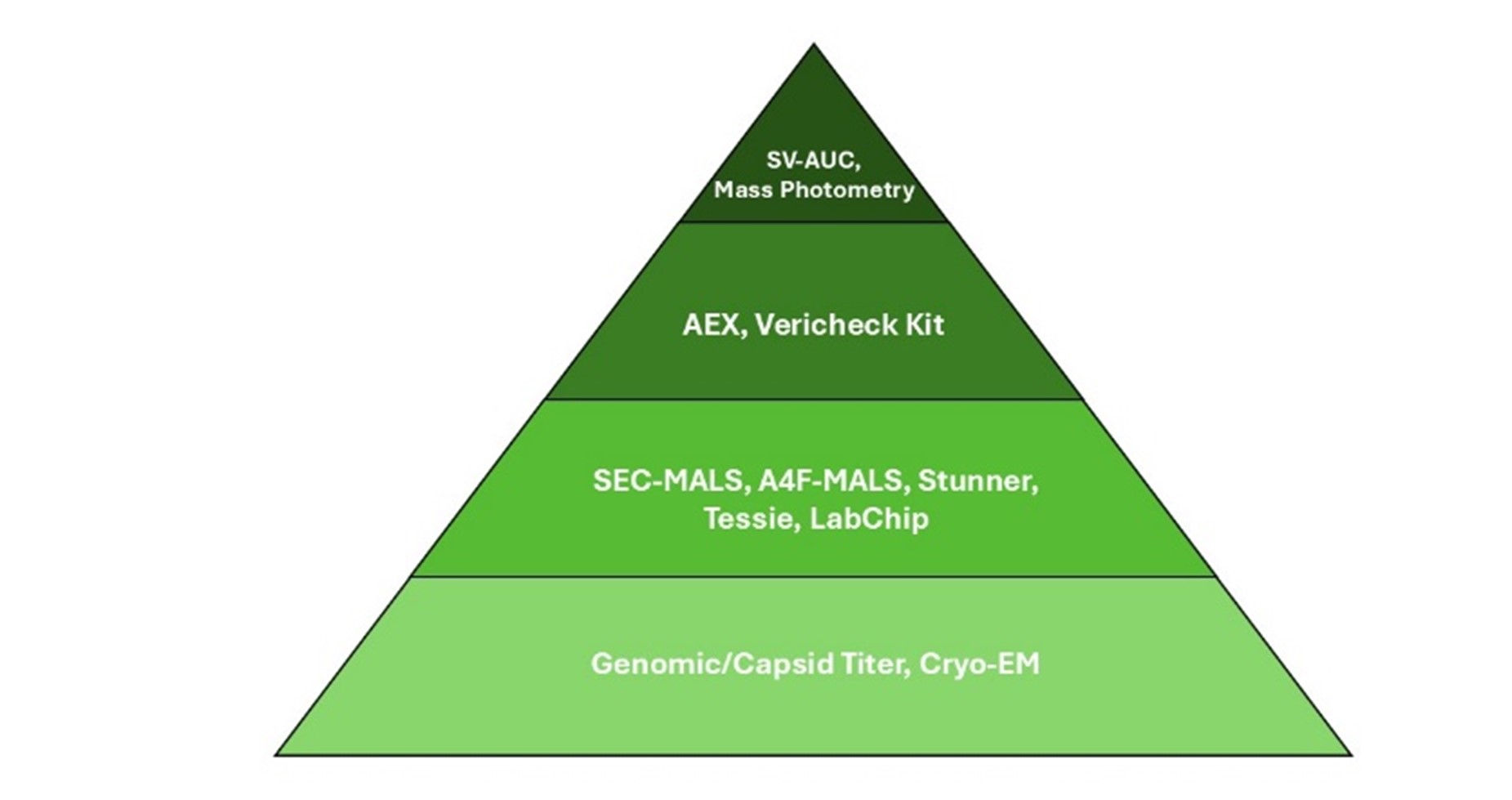
The image below ranks from least sensitive at the bottom to most sensitive at the pinnacle.
Questions to answer before making a choice of analytical methods for measuring percent empty/full capsids:
- What type of sensitivity are you trying to achieve? In certain laboratory scenarios, accuracy is regarded as beneficial rather than essential. Analytical methods that rely on input values, relative measurements, or ratios such as the cargo DNA to capsid ratio for determining percent full can be utilized. For more sensitive applications, methods like Tessie, Stunner, and commercially available kits are suitable. For extremely sensitive requirements, advanced methods such as SV-AUC, MP, SEC, and AF4 MALS are recommended.
- What resources do you have to achieve analytical goals? Analytical assays can sometimes be costly, such as SV-AUC, mass photometry, and MALS, as they require specialized instruments and trained personnel to operate. In such cases, it is crucial to evaluate the budget available for acquiring complex instruments. Alternatively, selecting a technique capable of assessing multiple CQAs on a single platform, such as SEC/AF4-MALS, Tessie, Stunner, or LabChip, might be a practical approach.
- What is the acceptable analysis time? Certain assays, such as SV-AUC and cryo-EM, have extended runtimes ranging from three to five hours, while others, like genomic titer/capsid titer, LabChip, Vericheck kits, and Tessie, require outputs from two distinct assays and may take one to two days to complete. For situations requiring rapid results, these analytical methods may not be ideal. In such cases, faster techniques like AEX and MP can be utilized effectively.
- What is the sample testing throughput? During process development, the number of samples tested may range from 70 to 300, depending on the specific type of development work. In high-throughput settings, employing a robust assay characterized by low variance and high reliability has proven transformative. A notable example is mass photometry, which has gained recognition for its effectiveness in measuring the percentage of full capsids.
- What type of material do you intend to test? Drug developers often test various sample types, including crude materials with high host cell impurities, process intermediates containing elevated salt levels, low pH, and other process-related impurities, as well as drug products that may include additives. Therefore, it is crucial to select analytical methods that are independent of the matrix in which the AAVs are present and to implement strategies to modify the matrix without introducing bias.
- Is it acceptable to add input values to produce an output? Some analytical methods rely heavily on theoretical input measurements, such as the extinction coefficient of the capsid, cargo, and cargo length. These input values are used to compute the output results, and alterations to these inputs can influence the outcomes. Methods like SEC-MALS, AF4-MALS, and Stunner are significantly dependent on these input parameters.
- How do you choose between absolute quantification and relative quantification? Certain analytical methods, such as SV-AUC, AEX, MP, and Vericheck kits can provide absolute readouts. However, others may use a standard curve to determine output values. In these cases, the process of establishing a reference standard and transitioning from an old reference standard to a new one can present challenges.
- What is the final fate of the assay? Drug developers often aim to transfer assays to GMP facilities. Quality control laboratories prefer well-characterized, robust, and easy-to-perform assays. Additional key considerations when selecting a platform for analytical testing include the ease of equipment qualification, setting acceptance criteria, training and knowledge transfer, and conducting risk assessments. Techniques such as SV-AUC and MP are particularly suited for quality control purposes.
These are the author's viewpoints. They are not associated with his current employer.
About The Author:
 Rishi Ramesh Kothari is a process development engineer at Regeneron Pre-Clinical Manufacturing and Process Development team focusing on development of analytical assays and achieving process development goals for Adeno-associated viruses going into clinic. He received his master’s degree in biotechnology from Drexel University College of Medicine.
Rishi Ramesh Kothari is a process development engineer at Regeneron Pre-Clinical Manufacturing and Process Development team focusing on development of analytical assays and achieving process development goals for Adeno-associated viruses going into clinic. He received his master’s degree in biotechnology from Drexel University College of Medicine.
