The Incredible Shrinking Laboratory -- Flow Cytometry on a Chip

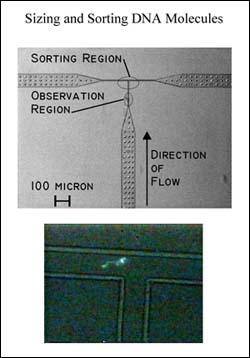
The µFACS uses electro-osmotic flow to move cells or particles along 3 µm-channels etched in a silicone-elastomer chip. Mounted on an inverted microscope, the chip is excited by a laser beam focused on the channel near a T-shaped junction (see figure). The signal is collected by a PMT, and once a threshold fluorescence level is recorded, the cell is diverted by a shift in the voltage to a collection well.
The researchers report several examples of sorting done with their petite FACS—a mixture of red and blue fluorescent beads, 1 µm in size, were sorted into single color pools with nearly 100-fold enrichment. In addition, live E. coli expressing green fluorescent protein mixed with various amounts of wild type E. coli were enriched 30-fold through sorting. Although they report the viability of the sorted E. coli at only 20%, as defined (number of colonies/number of fluorescent events recorded) this figure folds recovery into viability, and hence, viability may be better than it appears.
The µFACS offers a number of advantages beyond the obvious. It requires tiny amounts of material—the detection volume is in the femtoliter range—which not only saves on material, it also reduces the amount of background fluorescence, allowing greater sensitivities. A number of the technically troubling aspects of flow cytometry—droplet creation, aerosol formation, sterilization, and cross-contamination—are not an issue with this disposable FACS. In addition, it can be easily configured for parallel sorting, which will make it faster and allow sorting on multiple parameters.
For more information: Stephen R. Quake, Department of Applied Physics, California Institute of Technology, Pasadena, CA 91125. Email: quake@its.caltech.edu
By Laura DeFrancesco
