The Complex Regulatory Landscape Of Flow Cytometry For Cell Therapy
By Caraugh Jane Albany, Ph.D., Autolus Therapeutics

Flow cytometry enables both quantitative and qualitative analysis of cellular populations, evaluating properties such as relative size via forward scatter (FSC), complexity or granularity via side scatter (SSC), and marker expression via median fluorescence intensity (MFI). Flow cytometry is ubiquitously used in cell and gene therapy manufacturing and development for phenotypic and functional assessment of drug products throughout their life cycle. During manufacturing, flow cytometry is used to establish and monitor critical quality attributes (CQAs) and subsequent batch release.
Ensuring that flow experiments are adequately controlled is crucial to achieving reproducible and accurate data, particularly as there is an absence of international reference standards for this method. Background fluorescence, debris, poorly titrated antibodies, incorrect compensation, and incorrect gating can all cause variability in the obtained results. Assay developers must be meticulous from the start, given that even minor differences in execution can have significant implications. Technological advances in the field have increased the number of parameters that can be measured simultaneously, allowing for more detailed cell analysis.
This, coupled with advances in artificial intelligence (AI) and its integration into existing platforms such as the "elastic gating" feature on the BD FACSLyric flow cytometry system, presents further and emerging challenges for assay developers and regulators alike. Concurrently, regulatory agencies have placed increased scrutiny on flow cytometry-based approaches. This is evidenced by the publication of the Considerations for the Development of Chimeric Antigen Receptor (CAR) T Cell Products Guidance for Industry document in January 20242, which suggests including details such as assay controls, instrument calibration, QC activities, and gating strategy/panel design in the initial IND submission. Hinting at the necessity for quality by design (QbD) principles from assay conception, these guidelines are broad, requiring scientists to future-proof their flow-based assays with adequate controls and develop additional in-house policies and tools to ensure data integrity.
Futureproofing Now Could Save Time And Money Later
The development and validation of fit-for-purpose analytical flow-based assays poses a significant challenge for cell and gene manufacturing professionals. Often working under tight timelines, especially during early development, with limited pathologically relevant samples, they must also contend with complex and sensitive machinery. This can lead to an overreliance on machine-led QC steps without adequate human assessment. As such, in early clinical stages, where there’s often less stringent oversight or the need for a fully validated assay, the temptation might be to skip extensive optimization and testing steps before utilizing assays. Data from these poorly developed early-phase assays often lacks the rigor required for use in agency filings, and biologically relevant samples are limited and so should not be squandered.
Both American and European regulatory agencies have increasingly focused on key optimization and development steps for flow-based assays. This heightened scrutiny can be expected to grow. Thus, future-proofing assay design by adhering to and surpassing regulatory guidelines in the early stages can prevent costly redevelopment later. Companies should invest their time and effort in well-thought-out development of the flow-based methods, ensuring adequate time for essential steps such as panel design, clonal selection, antibody titrations, gating strategy development, and producing an accompanying guide and reportable parameter determination.
Using compensation as an example, we see increased attention has been given by regulatory agencies; for example, the Advanced Therapy Medicinal Products Guidance Application of Flow Cytometry (Section 7.2.7) states that "compensation is performed regardless of the number of fluorochromes used and should be assessed regularly."3 Similarly, FDA draft guidelines2 now require the initial IND submission to include assay controls, including compensation.
Compensation corrects the promiscuity of fluorophores, based on the principle that the degree of signal spillover into secondary detectors is directly proportional to the signal in the primary detector. However, compensation is often overlooked and undervalued during flow cytometry assay development, being seen as a necessary evil rather than an integrated assay step. When generating compensation controls, four key rules must be adhered to4:
- The staining of the compensation control must be as bright as or brighter than the sample.
- The compensation algorithm must be performed with both a positive and a negative population.
- The compensation control must use the same fluorophore as the sample.
- Enough events must be collected for the software to make a statistically significant determination of spillover (approximately 5,000 events for both the positive and negative populations).
Compensation wizards, which facilitate the automated generation of assay-specific compensation matrices, are used prolifically and can introduce variation in two ways:
- Generation of suboptimal compensation controls.
- Incorrect gate placement.
Cytometers will accept compensation assuming there is adequate resolution of the positive and negative populations but cannot discern the quality of the sample used or the gate placement. Given the increased attention on compensation, companies should instigate the use of thorough standardized operating procedures (SOPs) for preparing compensation controls, assessing, and using beads rather than cells for both viability and, where appropriate, CAR marker compensation to avoid variation. Attention should be placed on gate placement to ensure that the first rule of compensation is maintained where compensation control must be as bright as or brighter than the sample.4
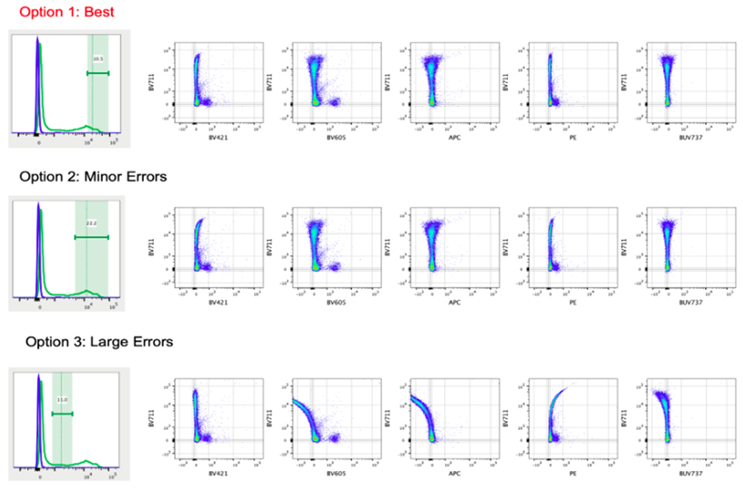
Figure 1: Gate placement has a dramatic effect on the compensation matrix generated. The same FCS file is used throughout this figure, but the compensation matrix has been varied across the three options. Option 1 has the best compensation matrix applied; it was generated by ensuring the gate placement only included the brightest positive event ensuring the dimmest shoulder of the peak was excluded. The matrix shown in option two shows some minor errors and was generate by gating the whole positive peak thus including the "dimly" positive shoulder region events. In the third option large errors (both overcompensation and undercompensation) can be seen in the plots. The matrix was generated incorrectly gating the region between the positive and negative populations. Figure adapted with permission from https://voices.uchicago.edu/ucflow/2020/06/24/the-right-and-wrong-way-to-set-up-automated-compensation-tools-how-to-achieve-accurate-compensation (Accessed Sept. 2024).5
Conventional sample-like gate placement (Option 2 in Figure 1) results in the inclusion of dimly positive compensation events, breaking the first rule and allowing errors in the compensation matrix. Instead, only the brightest events (Option 1 in Figure 1) should be used for compensation. As a rule of thumb, the compensation matrix can be considered correct when the MFI of the non-target channel is the same in the positive and negative population creating linear populations in bivariant data, i.e., when plotting PE against APC, for PE+ and PE- populations we would expect to obtain a similar MFI value for APC in correctly compensated samples. Data "frowning" toward the axis is overcompensated and data "smiling" toward the center of the plot is undercompensated.
Wherever possible, following suitable verification,3 the use of compensation beads over cells can help address variation in gate placement and help to homogenize the compensation matrixes generated as these bead-based reagents typically produce narrow fluorescent peaks, making gate placement easier. Compensation beads can be used to replace cell-based methods that have been historically used for viability dyes and anti-idiotype antibodies, which typically show higher levels of variability, so they present a risk factor for assay execution. In doing so companies would also remove the need to either qualify an internally manufactured lot of, or purchase and validate commercially available cells for the purpose of performing compensation, both of which are expensive and present a supply-chain risk.
Furthermore, in-house trending of the compensation matrix and visual inspection of N-by-N plots can be utilized as a troubleshooting tool to monitor for abnormalities in the compensation of a given assay. Implementation of such tools is alluded to in the Advanced Therapy Medicinal Products Guidance Application of Flow Cytometry (section 7.3.3): “The instrument should be regularly monitored for PMT drift, and a policy developed to adjust voltages and check compensation accordingly.”3 Similarly, additional focus can be seen on experiment optimization in the new guidelines, with titrations being specifically mentioned for the first time in Considerations for the Development of Chimeric Antigen Receptor (CAR) T Cell Products Guidance for Industry: “We recommend performing antibody titration to determine the optimal antibody dilution.”2
To draw quantitative conclusions from flow cytometric data, the fluorescence intensity of a given fluorophore must be directly and linearly proportional to the antigen being measured. This necessitates the use of reagent concentrations that are not limiting, ensuring that the staining intensity of a cell correlates proportionally to the antigen amount on the cell surface. The optimal concentration of a given antibody for flow cytometry depends on several key factors, including staining time, antibody volume, and cell concentration. Therefore, titration must be performed specifically for the intended sample density under the assay's staining conditions; performing this at assay conception and during development can prevent the need for costly revisiting.
QBD Future-minded Expectations For Operators Are Critical
Implementing QbD and allocating sufficient time and resources during early stages of analytical assay establishment can be challenging, from the generation of the materials themselves in the case of monoclonal antibodies for rare markers and the generation of specific anti-idiotype antibodies in the case of CAR T cell therapy, to the sometimes-prohibitive cost of purchasing and maintaining a 21 CFR Part 11c compliant multi-parameter cytometer. Execution of high-quality flow cytometry-based assays is undeniably costly.
Where possible, pharmaceutical companies should keep key assay aspects, including panel design, critical reagents, and cytometer used, consistent through all assay iterations to facilitate replicable comparisons to be drawn through the life cycle of the drug and to provide a stable framework for which modulations can occur, reducing costs. Furthermore, the actual execution of flow cytometry protocols can be a complex and sensitive methodology with a steep learning curve for those new to its execution.
Therefore, developers and companies are advised to provide training to operators under the expectation that operators will not only be executing assays but also:
- Undertaking machine maintenance.
- Preparing compensation controls.
- Gating populations.
Additionally, implementation of in-house compensation trending, N-by-N plot visualization, and bead controls for determining master mix content can serve as additional controls, aiding the detection of abnormalities and ensuring adherence to regulatory agencies’ guidance. Batch-to-batch variability monitoring should be ongoing and continually feed back into assay optimization during early development stages to ensure the end assay is fit-for-purpose. For example, normal operating range (NOR) and proven acceptable range (PAR) cell densities will need to feedback into the titrations performed to ensure assay conditions are optimal. Due to the limited availability of patient material, healthy donor material is often used during assay development. However, as healthy donor material may lack the same impurities, wherever possible, excess patient samples should be retained and pathological mimetics should be created to better assess flow assay performance under the intended test conditions.
Regulatory Scrutiny Of Flow Cytometry Is Expected To Grow
In conclusion, despite being a relatively new technology, multi-parameter flow cytometry is an indispensable technique that is used extensively in cell and gene therapy. Regulatory agencies are placing increasing emphasis on ensuring robust assay development. This scrutiny will only grow, highlighting the need for meticulous planning and design. Herein, we used compensation and antibody titration as an example; however, this logic can be applied to a wide range of design and development steps.
The gravity of not adhering to guidelines should not be underestimated; for context, statistics show that 84.5% of FDA-issued refuse-to-file letters (RFL) to drug application (between 2008 and 2017) were due to scientific deficiencies. The biggest portion were related to the chemistry, manufacturing, and controls (CMC),6 including lack of proper controls.7 Receiving an RFL is extremely costly to developers, with approval of resubmitted applications taking 16 to 18 months longer (in 2018) than the overall approval time for all new drug applications and biologic licensing applications (BLAs) reviewed.7 I speculate the cost of that is far higher than the cost of correct assay development during early phase. These findings highlight the importance of developers adhering to current guidelines and keeping abreast of developments in the field. By adhering to stringent regulatory guidelines and employing QbD principles from conception and beyond, the risk of regulatory rejection can be mitigated, ensuring the reliability of assays, and, ultimately, enhancing the safety and efficacy of therapeutic products for our patients.
References:
- Allison Irvine, Mohamed Mahmoud Moustafa, Sahul Patel et al. Automation of Flow Cytometry Data Analysis with Elastic Image Registration, 12 February 2024, PREPRINT (Version 1) available at Research Square [https://doi.org/10.21203/rs.3.rs-3912020/v1]
- U.S. Department of Health and Human Services, FDA, CBER (January 2024). “Considerations for the Development of Chimeric Antigen Receptor (CAR) T Cell Products, Guidance for Industry”. https://www.fda.gov/media/156896/download
- The British Pharmacopeia, MHRA (2022). “British Pharmacopoeia ATMP Guidance Application of Flow Cytometry”. https://www.pharmacopoeia.com/download/atmpguidance
- Bio-Rad (no date) Single Staining & Compensation Controls - flow cytometry guide: Bio-rad, Bio. Available at: https://www.bio-rad-antibodies.com/flow-cytometry-comp-controls.html (Accessed: 10 October 2024).
- Figure adapted with permission from: Johnston, L. (2024) The Right and Wrong Way to Set Up Automated Compensation Tools: How to Achieve Accurate Compensation, Cytometry and antibody technology. Available at: https://voices.uchicago.edu/ucflow/2020/06/24/the-right-and-wrong-way-to-set-up-automated-compensation-tools-how-to-achieve-accurate-compensation/ (Accessed: 10 September 2024).
- Chahal HS, Mukherjee S, Sigelman DW, Temple R. Contents of US Food and Drug Administration Refuse-to-File Letters for New Drug Applications and Efficacy Supplements and Their Public Disclosure by Applicants. JAMA Intern Med. 2021 Apr 1;181(4):522-529. doi: 10.1001/jamainternmed.2020.8866. PMID: 33587091; PMCID: PMC7885096.
- Karlin-Smith, S. (2021) FDA refuse-to-file decisions are rare, but CMC and ignoring agency advice are often triggers, Pink Sheet. Available at: https://pink.citeline.com/PS143806/FDA-RefuseToFile-Decisions-Are-Rare-But-CMC-And-Ignoring-Agency-Advice-Are-Often-Triggers (Accessed: 01 October 2024).
About The Author:
 Caraugh Albany is a senior scientist in the analytical development team at Autolus Therapeutics. She completed her Ph.D. at King's College London, focusing on the role of regulatory T cells in atherosclerosis, where she developed both functional and phenotypic flow-based assays for primary human T cells, B cells, and monocytes. In her current role, she continues to leverage her extensive flow cytometry experience in a GMP setting. You can find more about her work and background on her LinkedIn profile here.
Caraugh Albany is a senior scientist in the analytical development team at Autolus Therapeutics. She completed her Ph.D. at King's College London, focusing on the role of regulatory T cells in atherosclerosis, where she developed both functional and phenotypic flow-based assays for primary human T cells, B cells, and monocytes. In her current role, she continues to leverage her extensive flow cytometry experience in a GMP setting. You can find more about her work and background on her LinkedIn profile here.
