The Booming Merger Of Biopharma Separation Techniques & Mass Spectrometry
By Dan Bach Kristensen, principal scientist, Symphogen

Biopharmaceutical molecules, such as a classical monoclonal antibody (mAb), are inherently complex. A typical mAb consists of approximately 1,300 amino acids, which can undergo a broad range of co- and post-translational modifications.1,2 Other biopharmaceutical modalities, such bispecific antibodies and fusion proteins, can have an even higher level of molecular complexity.2
An overview of known modifications can be seen in the Unimod database, which to date contains over 1,500 modifications, many of which apply to protein pharmaceuticals. For instance, well-known variants or modifications of a classical mAb include glycoform variants, terminal variants (N-terminal pyroGlu, C-terminal Lys, loss C-terminal Gly followed by amidation), oxidation, deamidation, succinimide formation, and isomerization.
The combination of large molecular size and the large number of theoretical modifications leads to a combinatorial explosion of possible product variants, also known as proteoforms.3 Indeed, a biopharmaceutical product should be seen as a population of many molecular variants or proteoforms, which may vary from one production lot to another (e.g., in the glycoform distribution), and which may change over time through stability indicating modifications (e.g., deamidation, oxidation, isomerization).2 Regulatory bodies, such as the FDA and European Medicines Agency, require that product variants with altered safety or efficacy profiles are identified and controlled as part of the analytical control strategy for a biopharmaceutical product. The characterization and identification of biopharmaceutical proteoforms, including the critical proteoforms impacting safety and efficacy, pose significant analytical challenges for the reasons stated above. Fortunately, the last decade has seen a strong development in analytical tools used for physicochemical characterization of biopharmaceuticals. The technological development has to a large extent been fueled by the evolution of modern mass spectrometry (MS) as well the hyphenation of MS to a broad range of analytical separation techniques used in biopharmaceutical development.
The Great Impact of MS in Biopharmaceutical Development
MS technology has evolved to become an indispensable analytical aid throughout the development stages of a biopharmaceutical product, ranging from early discovery and developability assessment, through to late-stage development, regulatory filings, and current good manufacturing practices (cGMP) testing.4-7 For instance, characterization of critical quality attributes that impact safety and efficacy of biopharmaceuticals is now routinely performed using MS.5
The last decade has seen a multitude of separation techniques hyphenated to MS for top-down, middle-up, and bottom-up workflows; this has had a profound impact on the way biopharmaceutical characterization is performed.5 Of particular importance has been the introduction of native MS workflows, which employ front-end separation techniques in which proteins are maintained in a native, folded conformation during separation and electrospray ionization.8-10
Native MS holds great promise for studying protein structure-function relationships, such as the analysis of protein ligand interactions.11,12 Likewise, native affinity MS holds great promise for studying the structure-function relationship of biopharmaceutical proteoforms, as exemplified by the study of Lippold et al., in which native FcɣRIIIa affinity MS demonstrated differential binding affinity of different glycoforms to the FcɣRIIIa receptor.13 Similarly, native MS is now well established as a tool for characterizing biopharmaceutical proteoforms at the intact level. Both denaturing and native intact MS workflows, such as reversed-phase chromatography (RPC) MS, size-exclusion chromatography (SEC) MS, and cation-exchange chromatography (CIEX) MS, are now performed routinely in biopharmaceutical development.14,15 As a tool for characterizing proteoforms, native MS has the advantage of simpler mass spectra, containing fewer charge states as well as a lower number of charges, resulting in better spatial separation of charge states and overall higher peak capacity (Figure 1).
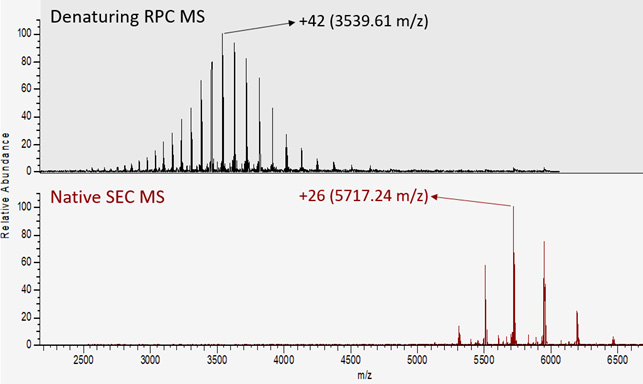
Figure 1: Denaturing versus native mass spectrometry (MS) of 150 kDa IgG1. Reversed-phased chromatography (RPC) disrupts non-covalent interactions (i.e., denaturation), resulting in high charge states (most intense is +42) and many charge states (over 20). In contrast, during size exclusion chromatography (SEC) MS, non-covalent interactions are preserved (native conformation), resulting in lower charge states (most intense is +26) and fewer charge states (around 6). Overall, native MS results in simpler spectra with higher spatial separation between charge states and higher peak capacity.
Hyphenating Biopharma Separation Techniques To MS
This ability to hyphenate a broad range of separation techniques to MS has had a profound effect on biopharmaceutical development laboratories (Table 1 below). For instance, the quality of a biopharmaceutical product in clinical development is controlled by a product specification, which is a list of analytical methods used for release and stability testing of a biopharmaceutical drug under cGMP regulations.16,17
Table 1: Examples of Separation Techniques Hyphenated to MS for Biopharmaceutical Characterization
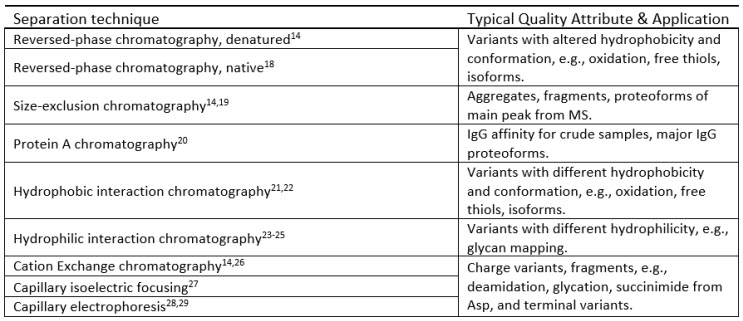
The specification typically contains a number of classical (non-MS) impurity methods (e.g., chromatography- or electrophoresis-based), which resolve and quantitate impurities based on relative peak areas (typically from UV trace). Ultimately, as a biopharmaceutical project progresses through clinical development, all impurities monitored by the GMP specification methods will need to be identified and characterized.
Since virtually all separation methods now can be hyphenated to MS, the task of characterizing impurities can be performed with great efficiency, speed, and sensitivity using online MS detection, which provides highly specific molecular masses of the individual impurities, thus revealing the chemical nature of the impurity.5
The near future is also likely to see a further migration of MS into cGMP environments.30 To date, cGMP testing of biopharmaceuticals by MS has evolved around the multi-attribute method (MAM), which employs a bottom-up approach, i.e., qualitative and quantitative evaluation of quality attributes at the peptide level by MS.7,31 Similarly, other MS workflows have a promising cGMP potential. One could, for instance, imagine intact MS analysis holds great potential as an identity (ID) test, since molecular mass can be determined to great accuracy at the intact level and requires no sample preparation. Similarly, intact MS can precisely quantitate major proteoforms (such as major glycoforms of an IgG) without any sample preparation, and this can be envisaged as a simple, yet powerful, quantitative MS tool for cGMP testing.
As the number of separation techniques hyphenated to MS continues to grow and the MS technology itself evolves, the influence of MS is expected to grow further at all stages of biopharmaceutical development.
References
- A. Beck, E. Wagner-Rousset, D. Ayoub, A. van Dorsselaer, S. Sanglier-Cianférani, Characterization of Therapeutic Antibodies and Related Products, Anal Chem. 85 (2013) 715–736. https://doi.org/10.1021/ac3032355.
- Y. Xu, D. Wang, B. Mason, T. Rossomando, N. Li, D. Liu, J.K. Cheung, W. Xu, S. Raghava, A. Katiyar, C. Nowak, T. Xiang, D.D. Dong, J. Sun, A. Beck, H. Liu, Structure, heterogeneity and developability assessment of therapeutic antibodies, MAbs. 11 (2019) 239–264. https://doi.org/10.1080/19420862.2018.1553476.
- L.M. Smith, N.L. Kelleher, T.C. for T.D. Proteomics, Proteoform: a single term describing protein complexity, Nat Methods. 10 (2013) 186. https://doi.org/10.1038/NMETH.2369.
- I. Apostol, P. v Bondarenko, D. Ren, D.J. Semin, C.-H. Wu, Z. Zhang, C.T. Goudar, Enabling development, manufacturing, and regulatory approval of biotherapeutics through advances in mass spectrometry., Curr Opin Biotechnol. 71 (2021) 206–215. https://doi.org/10.1016/j.copbio.2021.08.001.
- S. Liu, B.L. Schulz, Biopharmaceutical quality control with mass spectrometry., Bioanalysis. 13 (2021) 1275–1291. https://doi.org/10.4155/bio-2021-0123.
- I. Sokolowska, J. Mo, F. Rahimi Pirkolachahi, C. McVean, L.A.T. Meijer, L. Switzar, C. Balog, M.J. Lewis, P. Hu, Implementation of a High-Resolution Liquid Chromatography–Mass Spectrometry Method in Quality Control Laboratories for Release and Stability Testing of a Commercial Antibody Product, Analytical Chemistry. 92 (2020) 2369–2373. https://doi.org/10.1021/acs.analchem.9b05036.
- S. Rogstad, H. Yan, X. Wang, D. Powers, K. Brorson, B. Damdinsuren, S. Lee, Multi-Attribute Method for Quality Control of Therapeutic Proteins, Analytical Chemistry. 91 (2019) 14170–14177. https://doi.org/10.1021/acs.analchem.9b03808.
- I.K. Webb, Recent technological developments for native mass spectrometry., Biochim Biophys Acta Proteins Proteom. 1870 (2022) 140732. https://doi.org/10.1016/j.bbapap.2021.140732.
- S. Tamara, M.A. den Boer, A.J.R. Heck, High-Resolution Native Mass Spectrometry, Chemical Reviews. 122 (2022) 7269–7326. https://doi.org/10.1021/acs.chemrev.1c00212.
- A.C. Leney, A.J.R. Heck, Native Mass Spectrometry: What is in the Name?, J Am Soc Mass Spectrom. 28 (2017) 5–13. https://doi.org/10.1007/s13361-016-1545-3.
- G. van Schaick, R. Haselberg, G.W. Somsen, M. Wuhrer, E. Domínguez-Vega, Studying protein structure and function by native separation–mass spectrometry, Nature Reviews Chemistry. 6 (2022) 215–231. https://doi.org/10.1038/s41570-021-00353-7.
- J.L. Bennett, G.T.H. Nguyen, W.A. Donald, Protein-Small Molecule Interactions in Native Mass Spectrometry., Chem Rev. 122 (2022) 7327–7385. https://doi.org/10.1021/acs.chemrev.1c00293.
- S. Lippold, S. Nicolardi, E. Domínguez-Vega, A.-K. Heidenreich, G. Vidarsson, D. Reusch, M. Haberger, M. Wuhrer, D. Falck, Glycoform-resolved FcɣRIIIa affinity chromatography–mass spectrometry, MAbs. 11 (2019) 1191–1196. https://doi.org/10.1080/19420862.2019.1636602.
- D.B. Kristensen, T.M. Sloth, M. Ørgaard, P.F. Jensen, Characterization of Protein Glycoforms at Intact Level by Orbitrap Mass Spectrometry., in: A. Delobel (Ed.), Mass Spectrometry of Glycoproteins (Clifton, N.J.), Springer, 2021: pp. 23–45. https://doi.org/10.1007/978-1-0716-1241-5_2.
- F. Füssl, L. Strasser, S. Carillo, J. Bones, Native LC–MS for capturing quality attributes of biopharmaceuticals on the intact protein level, Current Opinion in Biotechnology. 71 (2021) 32–40. https://doi.org/10.1016/j.copbio.2021.05.008.
- International Council for Harmonisation, ICH Guideline Q6B - Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products, ICH Harmonised Tripartite Guideline. (1999) 1–20.
- FDA, Facts About the Current Good Manufacturing Practices (CGMPs), (2022). https://www.fda.gov/drugs/pharmaceutical-quality-resources/facts-about-current-good-manufacturing-practices-cgmps (accessed June 7, 2022).
- T.H. Chen, Y. Yang, Z. Zhang, C. Fu, Q. Zhang, J.D. Williams, M.J. Wirth, Native Reversed-Phase Liquid Chromatography: A Technique for LCMS of Intact Antibody-Drug Conjugates, Analytical Chemistry. 91 (2019) 2805–2812. https://doi.org/10.1021/acs.analchem.8b04699.
- M. Haberger, M. Leiss, A.K. Heidenreich, O. Pester, G. Hafenmair, M. Hook, L. Bonnington, H. Wegele, M. Haindl, D. Reusch, P. Bulau, Rapid characterization of biotherapeutic proteins by size-exclusion chromatography coupled to native mass spectrometry, MAbs. 8 (2016). https://doi.org/10.1080/19420862.2015.1122150.
- C. Jakes, F. Füssl, I. Zaborowska, J. Bones, Rapid Analysis of Biotherapeutics Using Protein A Chromatography Coupled to Orbitrap Mass Spectrometry, Analytical Chemistry. 93 (2021). https://doi.org/10.1021/acs.analchem.1c02365.
- B. Chen, Z. Lin, A.J. Alpert, C. Fu, Q. Zhang, W.A. Pritts, Y. Ge, Online Hydrophobic Interaction Chromatography-Mass Spectrometry for the Analysis of Intact Monoclonal Antibodies., Anal Chem. 90 (2018) 7135–7138. https://doi.org/10.1021/acs.analchem.8b01865.
- B. Wei, G. Han, J. Tang, W. Sandoval, Y.T. Zhang, Native Hydrophobic Interaction Chromatography Hyphenated to Mass Spectrometry for Characterization of Monoclonal Antibody Minor Variants, Analytical Chemistry. 91 (2019) 15360–15364. https://doi.org/10.1021/acs.analchem.9b04467.
- Y. Zhao, S. Raidas, Y. Mao, N. Li, Glycine additive facilitates site-specific glycosylation profiling of biopharmaceuticals by ion-pairing hydrophilic interaction chromatography mass spectrometry, Analytical and Bioanalytical Chemistry. 413 (2021). https://doi.org/10.1007/s00216-020-03089-3.
- E. Domínguez-Vega, S. Tengattini, C. Peintner, J. van Angeren, C. Temporini, R. Haselberg, G. Massolini, G.W. Somsen, High-resolution glycoform profiling of intact therapeutic proteins by hydrophilic interaction chromatography-mass spectrometry, Talanta. 184 (2018). https://doi.org/10.1016/j.talanta.2018.03.015.
- V. D’Atri, S. Fekete, A. Beck, M. Lauber, D. Guillarme, Hydrophilic Interaction Chromatography Hyphenated with Mass Spectrometry: A Powerful Analytical Tool for the Comparison of Originator and Biosimilar Therapeutic Monoclonal Antibodies at the Middle-up Level of Analysis, Analytical Chemistry. 89 (2017). https://doi.org/10.1021/acs.analchem.6b04726.
- F. Füssl, K. Cook, K. Scheffler, A. Farrell, S. Mittermayr, J. Bones, Charge Variant Analysis of Monoclonal Antibodies Using Direct Coupled pH Gradient Cation Exchange Chromatography to High-Resolution Native Mass Spectrometry, Analytical Chemistry. 90 (2018) 4669–4676. https://doi.org/10.1021/acs.analchem.7b05241.
- J. Dai, J. Lamp, Q. Xia, Y. Zhang, Capillary Isoelectric Focusing-Mass Spectrometry Method for the Separation and Online Characterization of Intact Monoclonal Antibody Charge Variants, Analytical Chemistry. 90 (2018). https://doi.org/10.1021/acs.analchem.7b04608.
- R. Haselberg, V. Brinks, A. Hawe, G.J. de Jong, G.W. Somsen, Capillary electrophoresis-mass spectrometry using noncovalently coated capillaries for the analysis of biopharmaceuticals, Analytical and Bioanalytical Chemistry. 400 (2011). https://doi.org/10.1007/s00216-011-4738-4.
- J. Sastre Toraño, R. Ramautar, G. de Jong, Advances in capillary electrophoresis for the life sciences, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences. 1118–1119 (2019). https://doi.org/10.1016/j.jchromb.2019.04.020.
- D. Ren, Advancing Mass Spectrometry Technology in cGMP Environments., Trends Biotechnol. 38 (2020) 1051–1053. https://doi.org/10.1016/j.tibtech.2020.06.007.
- R.S. Rogers, N.S. Nightlinger, B. Livingston, P. Campbell, R. Bailey, A. Balland, Development of a quantitative mass spectrometry multi-attribute method for characterization, quality control testing and disposition of biologics., MAbs. 7 (2015) 881–890. https://doi.org/10.1080/19420862.2015.1069454.
About The Author:
 Dan Bach Kristensen is a principal scientist at Symphogen. He holds a Ph.D. in biology and B.Sc. degree in chemistry. Kristensen is specialized in protein chemistry and mass spectrometry, which he initially applied in the field of proteome research in Japan and later in Denmark. For the last 18 years, he has been working with analytical development in the biopharmaceutical industry, on projects ranging from early discovery to product registration. Clinical indications include bleeding disorders, neutropenia, autoimmune diseases, and oncology.
Dan Bach Kristensen is a principal scientist at Symphogen. He holds a Ph.D. in biology and B.Sc. degree in chemistry. Kristensen is specialized in protein chemistry and mass spectrometry, which he initially applied in the field of proteome research in Japan and later in Denmark. For the last 18 years, he has been working with analytical development in the biopharmaceutical industry, on projects ranging from early discovery to product registration. Clinical indications include bleeding disorders, neutropenia, autoimmune diseases, and oncology.
