TFF Offers Ideal Anaerobic, GMP Conditions For LBP Concentration
By Jimi Kjærsgaard Pettersson, NIRAS
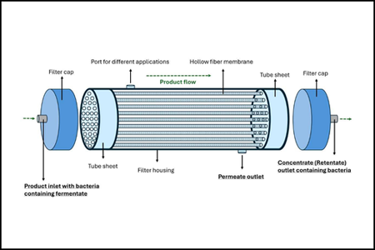
Live biotherapeutic products (LBPs) are an emerging class of therapeutics, where viability, purity, and manufacturing control are essential for product quality, especially with strict anaerobic microorganisms.
In such cases, oxygen exposure can damage metabolic pathways, reduce viability, or introduce unintended stress responses that ultimately affect clinical performance.
Within the upstream manufacturing workflow, the concentration of biomass after fermentation is one of the critical operational steps.
Concentration determines the final cell density, supports buffer exchange, and prepares the biomass for formulation and drying. At the same time, it represents a point where the risk of oxygen ingress, shear damage, and process variability is high.
For anaerobic LBPs, success depends on the ability to perform concentration within a closed, oxygen-controlled, GMP-compliant environment that maintains the functional integrity of the microorganisms.
Tangential flow filtration (TFF), also known as cross-flow filtration, has emerged as the preferred method for concentrating LBPs due to its scalability, controlled hydrodynamics, and compatibility with single-use and closed-system operation.
When configured correctly, TFF provides the gentle processing environment and oxygen protection required for strict anaerobes, making it central to commercial LBP manufacturing.
This article explores the role of concentration in anaerobic LBP production, why TFF is uniquely suited for this purpose, and which engineering and GMP considerations are necessary to protect viability throughout the process.
The Role Of Concentration In LBP Manufacturing
The concentration step bridges fermentation and the downstream manufacturing stages where formulation, drying, and final dosage preparation occur.
Before the biomass can transition to these later steps, the fermentation broth must be reduced while maintaining cell viability, consistency, and purity.
Achieving The Required CFU Concentration
Most LBPs must reach a defined concentration range before being transferred to formulation or drying. Concentration increases biomass density without compromising cell integrity or altering the strain’s characteristics.
For anaerobes, the process must occur in a strictly oxygen-free environment to prevent changes to viability or metabolic state.
Reducing Fermentation Broth And Initiating The Transition Toward Formulation
During concentration, the process begins reducing the fermentation broth volume and initiating the controlled transition toward a formulation-ready environment.
At this point, only some of the remaining media components are removed; if necessary, they can be fully reduced or replaced with a defined formulation buffer later using closed, strictly anaerobic diafiltration.
Separating these steps protects viability, minimizes oxygen exposure, and ensures that the concentrated biomass meets GMP requirements for purity and consistency. Additionally, if diafiltration is not needed for a particular process, it is possible to proceed directly to subsequent manufacturing stages after concentration. This flexibility allows the process to be tailored to the specific requirements of the product, while still maintaining the necessary controls to protect cell viability and ensure compliance with purity and consistency standards.
Protecting Viability Under Gentle, Controlled Conditions
Anaerobic LBPs are sensitive to shear stress, oxygen exposure, pH shifts, osmotic changes, and elevated temperatures. Concentration must therefore provide a tightly controlled physical environment that supports microbial stability.
This is especially important for strict anaerobes, where even slight deviations can reduce viability dramatically.
Supporting GMP Compliance And Manufacturing Consistency
From a regulatory perspective, concentration is a key step where:
- in-process controls are applied,
- oxygen exposure risk is high,
- viability and purity are verified, and
- system integrity is maintained.
The ability to execute this step reproducibly and document each parameter can be essential for batch release and regulatory approval.
Why Tangential Flow Filtration Is Preferred For Anaerobic LBPs
Several methods exist for concentrating microbial biomass, including centrifugation and depth filtration. However, only a few provide the appropriate balance of shear control, oxygen protection, and GMP compliance required for strict anaerobes.
1. Gentle Hydrodynamics That Protect Fragile Cells
TFF operates by moving the feed stream parallel to the membrane surface, minimizing fouling and avoiding the direct impingement forces associated with traditional filtration.
Compared with centrifugation, which exposes cells to high g-forces, TFF maintains low and controlled shear levels.
Optimizing transmembrane pressure (TMP), cross-flow velocity, and membrane geometry ensures that even highly sensitive anaerobic strains can be processed without mechanical damage.
2. Closed, Oxygen-Controlled Operation
One of the primary reasons TFF is preferred for anaerobic LBPs is its compatibility with fully closed, oxygen-protected systems.
All process steps, from loading the biomass to transferring the concentrated product, can be executed using oxygen-impermeable tubing, sterile connectors, and nitrogen-blanketed vessels. This is essential for preventing viability loss caused by trace oxygen exposure.
3. Scalability From Development To Commercial Manufacturing
TFF systems can scale linearly from bench-scale development to multi-thousand-liter commercial manufacturing. Whether using hollow fiber modules or flat-sheet cassettes, membrane area can be increased without altering core process parameters.
Maintaining similar hydrodynamic profiles across scales supports robust process transfer and reproducibility.
4. GMP Compatibility And Regulatory Acceptance
TFF is widely used in GMP biologics manufacturing and systems are available with:
- documented material traceability,
- validated extractables and leachable data,
- integrity testing,
- CIP/SIP compatibility, and
- comprehensive automation and monitoring options.
This makes regulatory submissions more straightforward and provides assurance that the technology is suitable for producing a clinical or commercial LBP.
5. Selecting Between Hollow Fiber And Cassette Systems
Both technologies are suitable for anaerobic LBPs, but their performance characteristics differ. Hollow fiber filters offer smooth lumen geometries and low-shear environments ideal for fragile cells. Cassette-based systems excel in larger-scale operations and multi-product facilities due to their modularity and ease of cleaning or disposal.
The choice depends on the specific strain, shear sensitivity, scale, and facility design.
The following figures are an example of a hollow fiber and the associated process involved in concentrating fermentate from a strict anaerobe fermentation.
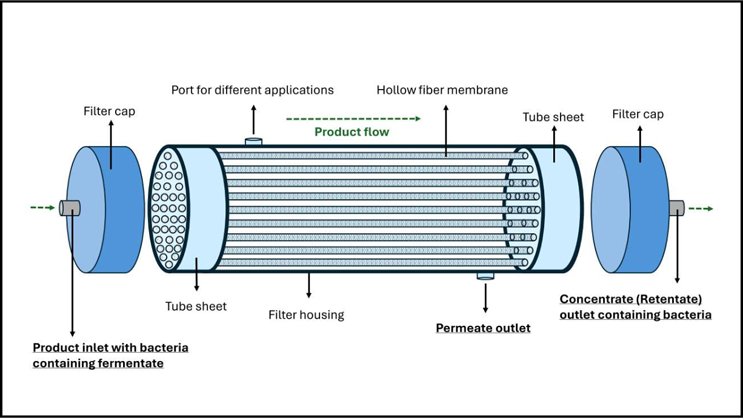
Source: Jimi Kjærsgaard Pettersson
Figure 1: A schematic representation illustrating the configuration of a hollow fiber system.
Figure 1 depicts a hollow fiber system used in the concentration of fermentate from strict anaerobe fermentation. In this schematic, the system consists of a series of hollow fiber modules arranged to allow fluid to flow through the lumens while retaining cells and larger particles within the fibers. The permeate, typically consisting of smaller molecules and buffer, passes through the fiber walls and is collected separately, enabling efficient concentration and washing of the target product under controlled conditions.
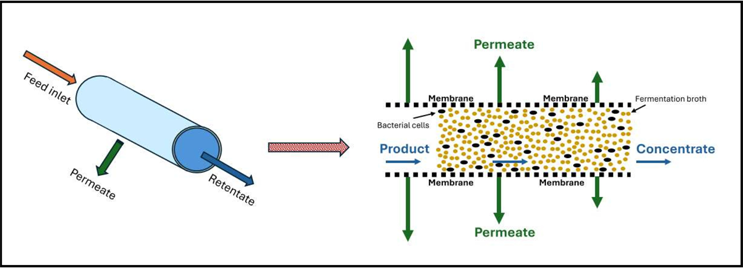
Source: Jimi Kjærsgaard Pettersson
Figure 2: The illustration presents an individual hollow fiber tube, detailing both its structure and the process dynamics occurring within the hollow fiber tube.
Figure 2 provides a closer look at an individual hollow fiber tube, highlighting its internal structure and the dynamics of the filtration process. The illustration shows how the fermentate enters the lumen, where fragile anaerobic cells are protected by the smooth geometry and low-shear environment. As the process continues, liquid is forced through the semi-permeable membrane wall, leaving concentrated cells inside the lumen while allowing unwanted components to be removed with minimal disturbance to cell integrity.
Engineering TFF Systems For Strict Anaerobic Conditions
Ensuring fully anaerobic conditions during concentration is the main engineering challenge for strict anaerobe-based LBPs. Oxygen can enter via tubing, leaks, headspace, or buffer additions, so all system aspects must be tightly controlled.
Oxygen Permeability Of Tubing And Flow Paths
Choosing the right tubing is essential, as standard materials like silicone and some TPEs allow significant oxygen diffusion during multi-hour TFF runs, risking cell viability. Fluoropolymer tubing (FEP, PFA, PTFE) or multilayer tubing with oxygen barriers is preferred for minimizing oxygen ingress in anaerobic processes.
Nitrogen Blanketing And Closed Headspace Management
To prevent oxygen from entering through vessel headspace, all tanks and reservoirs can be purged with nitrogen or another oxygen-free gas. Maintaining a slight positive pressure helps protect against backflow and atmospheric intrusion.
Blanketing systems must also be validated for sterility and stability, ensuring that pressure fluctuations do not compromise the anaerobic environment.
Deoxygenated Buffers And Redox Control
Buffers used for concentration and diafiltration must be fully deoxygenated before entering the TFF loop. This typically includes nitrogen sparging, inline degassing, and, when needed, the addition of reducing agents such as cysteine to support low redox potential.
Continuous redox monitoring ensures that reducing conditions remain within the defined range for the specific anaerobic strain.
Management Of Carbon Dioxide And Gas Evolution
Some anaerobic strains keep metabolizing during concentration, generating CO₂ that can build up in the system. Degassing, typically performed in the fermenter or buffer tank, along with back-pressure regulators and well-placed vents, is essential for removing excess gas, maintaining stable flow, and preserving anaerobic integrity without disrupting TMP.
Closed Sterile Connections
Every transfer, loading, concentration, buffer addition, and product discharge, must be performed using closed, sterilizable connection technologies.
Sterile welds, aseptic connectors, and single-use manifolds provide the necessary protection against oxygen ingress and microbial contamination.
System Preparation
The TFF system is assembled (plastic or stainless skid, or single-use), flushed with deoxygenated buffer, equilibrated under nitrogen, then baseline DO and redox readings are taken and sensors calibrated.
Anaerobic Biomass Transfer
Biomass is transferred directly from the fermenter or from a buffer tank into the TFF system using sterile, oxygen-impermeable welding or closed connectors.
Receiving vessels can be nitrogen-blanketed to prevent oxygen exposure.
Initial samples are taken for identity, CFU/mL, purity, and viability assessment.
Concentration
Cross-flow velocity and TMP are gradually brought to operating conditions.
Working volume decreases as permeate is generated, and the biomass becomes progressively more concentrated. Throughout the process, operators monitor inlet pressure, outlet pressure, TMP, pH, temperature, membrane flux, and, sometimes, periodic measurement of DO and redox potential, to ensure optimal performance.
In-Process Controls
During concentration, viability, CFU counts, purity, and microbial integrity can be monitored through predefined IPCs.
Transfer To Formulation Or Drying
Upon achieving the target concentration, the biomass is transferred to the downstream formulation or drying stage under strictly anaerobic conditions. All transfer activities must be thoroughly documented, including recordings of oxygen levels and results from environmental monitoring.
Diafiltration And Removal Of Residual Media Components
Following concentration, many anaerobic LBPs require diafiltration to remove the remaining broth components and transition the biomass into the formulation buffer that will support the final drying step.
In anaerobic manufacturing, this operation must be performed with the same level of oxygen control as fermentation and concentration, since the extended recirculation and buffer addition phases increase the risk of oxygen ingress.
Diafiltration serves several purposes. First, it reduces or eliminates residual nutrients, peptides, salts, and metabolic by-products that would otherwise interfere with long-term stability or affect the downstream drying process. Second, it adjusts the osmolarity and chemical environment of the biomass to match the requirements of the final formulation buffer.
In many processes, the diafiltration buffer already contains the cryo-formulation excipients or drying protectants that will be used in the next stage.
Incorporating these excipients directly into the diafiltration buffer eliminates the need for an additional buffer exchange later in the workflow, reduces handling steps, and minimizes exposure of the anaerobic biomass to further processing stresses.
The challenge lies in performing this exchange entirely under anaerobic conditions. All buffer vessels must be pre-sparged with nitrogen or an equivalent oxygen-free gas, and the flow path between the buffer tank and the TFF loop must be constructed from low-permeability tubing to prevent oxygen diffusion during the diafiltration cycle.
As each volume of fresh buffer is introduced, the system must maintain a reducing redox potential, and dissolved oxygen must be monitored to ensure that ingress has not occurred. Even a brief elevation in DO during diafiltration can have an impact on viability, particularly for extremely oxygen-sensitive strains.
From a GMP perspective, diafiltration is not simply a washing step; it is a controlled and validated operation. Manufacturers must demonstrate that the buffer exchange occurs as intended, that targeted media components are reduced below predefined limits, and that the anaerobic environment is maintained throughout.
This requires supporting data from development studies, inline monitoring, and batch records that document oxygen control, membrane performance, excipient transfer, and final biomass composition.
When executed correctly, diafiltration provides a clean and controlled transition from fermentation broth into the defined excipient system used for drying or formulation, enabling a consistent and high-quality LBP drug substance without compromising viability or stability.
About The Author:
 Jimi Pettersson is expertise director at NIRAS, with over 15 years of experience driving R&D and innovation in life science and pharmaceutical development. He has led the advancement and implementation of novel technologies for live biotherapeutic products, specializing in fermentation, downstream processing, and advanced drying techniques. Pettersson has a strong track record in cross-functional leadership, strategic collaborations with academia and industry, and IP management. He holds an M.Sc. in dairy technology and an Executive MBA from DTU, combining scientific expertise with leadership in the life sciences sector.
Jimi Pettersson is expertise director at NIRAS, with over 15 years of experience driving R&D and innovation in life science and pharmaceutical development. He has led the advancement and implementation of novel technologies for live biotherapeutic products, specializing in fermentation, downstream processing, and advanced drying techniques. Pettersson has a strong track record in cross-functional leadership, strategic collaborations with academia and industry, and IP management. He holds an M.Sc. in dairy technology and an Executive MBA from DTU, combining scientific expertise with leadership in the life sciences sector.
