SUREtechnology Platform™

Explore how an advanced Cell Line Development (CLD) platform for new biologics can enable CHO-based cell line development; ensure premium, stable, and high-yield results; overcome common expression bottlenecks; and provide robust verification, genomic characterization, and monoclonality assessment services.
An Advanced Cell Line Development (CLD) Platform For New Biologics
- CHO-Based Cell Line Development
- Premium, Stable, and High-Yield
- Overcomes common expression bottlenecks
- Robust Verification, Genomic Characterization, and Monoclonality Assessment Services
Innovation Meets Expertise With Our Premium Cell Line Development
Welcome To The Future Of Biologics Development.
The SUREtechnology Platform is designed to overcome common expression bottlenecks in cell line development. Through advanced gene technology, our platform streamlines the development process from transfection to a fully-characterized research cell bank (RCB).
We overcome the challenges of transcription, translation, DNA repair, secretion, protein folding, and glycosylation to bring you a premium suite of cell line development tools and technologies.
Defining Next-Generation CLD With The SUREtechnology Platform™
Streamlined, Advanced Cell Line Development Supporting Biopharmaceutical Innovation
Expect versatility and leading performance with our SURE CHO-M Cell Line™
Our proprietary SURE CHO-M Cell Line™ is a game-changer in biologics production. Derived from CHO-K1 cells, this high-performing cell line was specifically developed to overcome protein expression bottlenecks. It enables the expression of a diverse range of molecules with high titers, ensuring versatility, high performance, and stability over 60 generations.
Robust verification with genomic characterization and monoclonality assessment
In ensuring the safety and success of biotherapeutics, we perform extensive genomic characterization and monoclonality assessment, based on the full sequencing of our proprietary SURE CHO-M Cell Line™. Leveraging next-generation sequencing technologies such as Illumina sequencing, we provide the detailed genomic data that is crucial for cost-effective biomanufacturing, safe clinical trials, and overall program success. Our in-house genomic analysis technologies and proprietary SUREsignature™ services deliver comprehensive monoclonality assessments, and genomic characterization of your RCBs, providing the right level of assurance and detailed support for your regulatory filings.
Discover A World Of Advanced Cell Line Development
Our suite of tools and technologies is your solution for premium, stable, high-yield, and integrated CHO-based cell line development.
Extensive, Diverse Molecule Support - Across a wide range of molecules, including mAbs, bispecifics, Fc fusions, and more
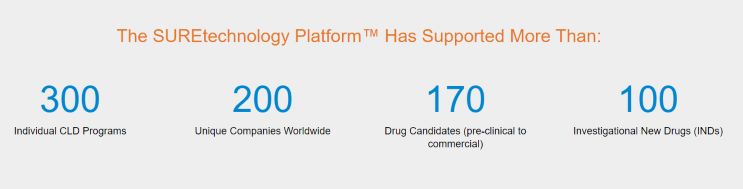
At KBI Biopharma, our SUREtechnology Platform has successfully supported the expression of a wide range of molecules for biopharmaceutical and vaccine development, coupled with robust verification to assure biosafety and efficacy. Across 300+ programs for 200+ global customers, we have supported the development of:
- 150+ monoclonal antibodies (mAbs)
- 40+ bispecific antibodies
- 35+ Fc fusions
- 40+ non-IgG molecules, including vaccines, blood factors, hormones, and enzymes, among others
Not Just Another CLD Platform - Our unique and patented technology enables faster, safer, and more efficient biopharmaceutical development.
Embedded in our transfection vectors are patented chromatin modifiers that unwind the DNA at the site of transgene integration, boosting transcription and therefore increasing recombinant protein expression. These epigenetic elements are key to obtaining the highest - and most reproducible - gene expression levels across protein types.
Furthermore, we believe in full transparency and robust verification. To that end, our extensive genomic characterization and monoclonality assessment services assure the biosafety and efficacy of developed products.
You've Met Your Match
KBI Biopharma is a trusted global CDMO with a strong track record offering best-in-class mammalian-based expression for breakthrough molecule types.
