6 Strategies To Protect Your Biologics Supply Chain In 2023
By Alexander K. Aust, M.S., MBA, Aust Business Solutions

Biopharmaceutical companies rarely have the luxury of extra time. So when supply chains stall — a more frequent reality in the post-pandemic era — it has ugly consequences for unprepared drug manufacturers. You can't afford to wait.
Drug makers are ripping up their old supply chain resilience strategies and rebuilding them. You might feel tempted to invest in automation, enchanted by the allure of technology managing everything. However, full automation is expensive, and it doesn't solve every supply chain problem.
Instead, focus on building scalable and need-specific supply chain operations. You should focus on the factors you can reasonably control. We'll discuss some of them here.
While it is not necessary to be fully automated, a high-performing supply chain must be fully integrated from sales and operations planning (S&OP) down to the day-to-day activities on the manufacturing floor.
For discussion, the flow chart in Figure 1 shows a high-level overview of a hypothetical process for bringing cGMP raw materials into a manufacturing facility. When you are calculating the total lead time of a cGMP raw material, it is not sufficient to simply take the difference between ordering and receiving to get the value. Why not? Because there are still seven major steps to complete before the cGMP material can be used to manufacture a batch, and each takes time. A manufacturer’s supply chain staff must understand each step of their process to allow for optimization and effective communication, both internally between departments and externally with partners.
Figure 1: Process overview for procurement of cGMP raw materials
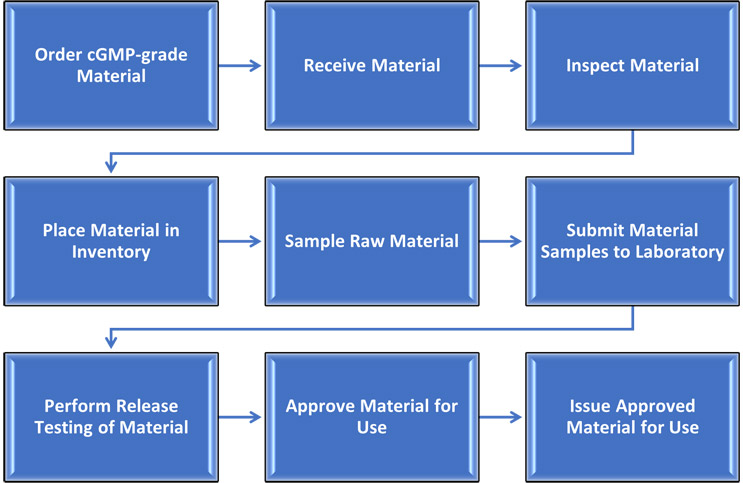
There are many ways to optimize a supply chain. To know which will make the biggest impact at your own organization, start by mapping your current supply chain processes so that you understand exactly how you are currently operating. Map each unit operation until all supply chain staff members have a full picture of the interconnectivity of all the departments and companies responsible for, directly involved in, and supporting manufacturing. Then, start the process of optimizing. Based on the process in Figure 1, the following is a list of tools and recommendations that can be introduced to decrease turnaround time, mitigate risk, and reduce cost.
Make Safety Stock Values Meaningful
Supply chain teams must keep an adequate amount of raw materials to meet the production schedule, and it all starts with S&OP. A Safety Stock is a safety net of material that protects the manufacturer from the ups and downs of demand. It may seem obvious, but when multiple Sponsors start requiring the use of the same materials as a CDMO grows, the Safety Stock for each material needs to be evaluated and reevaluated.
When evaluating what the Safety Stock should be, consider the Total Lead Time; set the Safety Stock at a value that allows you to meet the forecast while still leaving yourself enough time to replenish before a stockout occurs. Since the Supply Plan is a function of the Demand Plan, Sponsors must communicate their Demand changes as soon as possible.
Use A Foreign Trade Zone
In the pharmaceutical industry, a sponsor’s suppliers can be spread across countries and continents. By taking advantage of the Foreign Trade Zones Act of 1934, U.S.-based companies can turn their supply chain operations into a key differentiator and a strategic competitive advantage.
A foreign trade zone (FTZ) can be thought of as a free trade zone and is an area that operates outside of the typical requirements of U.S. Customs and Border Protection. It may sound a little too good to be true that a company can reduce lead time on an international raw material by bypassing customs inspections and FDA import systems, but it is true – to a certain degree.1 Using an FTZ has multiple benefits, as illustrated in Table 1.2
The biggest benefits can come when uncertainty is the highest, like during the beginning of an IND project and right before receiving approval of the NDA. However, this will not be an option for everyone, because the physical location of the FTZ matters to the U.S. government, and there can be significant costs. To best understand the requirements, risks, and benefits, consult an attorney that specializes in the FTZ field.
Table 1. Benefits of using a foreign trade zone

Ante Up With Electronic Management Systems
Electronic management systems are table stakes for a modern sponsor and competitive CDMO. Specifically, an electronic quality management system (eQMS), like Qualio or Veeva, helps track the deviations and statuses associated with each raw material, vendor, audit, and product. An enterprise resource planning (ERP) system helps control inventory levels, production values, and financial/accounting information on a validated system.
These electronic systems will help your entire company maintain greater control over operations and quality, not just supply chain. There are electronic systems available for companies of all sizes and built for all budgets, with the more robust systems being programs like SAP and NetSuite.
Be sure to spend enough of your project time and budget training the trainers and end users of each of these systems.
Anoint A Master Scheduler
Coordination between supply chain, project management, manufacturing, quality control, and quality assurance departments is of paramount importance when trying to keep the total lead time for cGMP raw materials low.
A dedicated master scheduler should report through the supply chain department, not manufacturing. The master scheduler and buyers must provide up-to-date information for the team members responsible for scheduling the release testing in the quality control laboratory. Some tests may be outsourced to a third-party lab and have greater lead times than in-house testing. This will allow quality control and quality assurance to make sure that instruments are available for use and the required prep can be performed in time for a batch that is on the production schedule.
Have A Clear Sampling Plan And Specification For Each Material
This all stems from supply chain staff working closely with quality control staff to understand the release testing requirements. Biologics and parenteral products are extremely expensive. Risk of contamination of the material and waste should be mitigated as much as possible, and this can be achieved by requesting a side sample of the material from the vendor. Communicate release testing requirements with the vendor and ask them to ship samples for release testing in a separate container from the material intended to go into a batch.
This bypasses the need for sampling in an environmentally controlled area altogether. You are also going to need an approved specification for quality assurance to perform their receipt and release functions. The sponsor and CDMO must both agree on the specification, and this, too, can be a cause for an increase in the total lead time.
Establish Phase-appropriate Processes With Risk-based Foundations
Since the goal is to reduce total lead time of a cGMP-grade material as much as possible, don’t waste valuable time with unnecessary release testing. For instance, if you are trying to introduce a lipid or a peptide into the cGMP area for an early-stage clinical drug product, testing requirements are less than the testing required for a commercial product.4
Additionally, based upon a thorough risk assessment and well-documented procedures, it may be acceptable to the sponsor, CDMO, and vendor to ship materials “at risk.” This means to move forward with a shipment before a cGMP material or product has been fully released, as indicated by the associated certificate of analysis (CoA).
This strategy assumes that by the time the material or product is used, it will have been approved and released without delaying the next operation.
About The Author:
 Alexander Aust, M.S., MBA, is the co-founder and owner of Aust Business Solutions, a pharmaceutical consultancy based in Maryville, Tennessee, where he focuses on strategic planning and partnerships, and technical support. The firm provides CMC and project management services, assists with regulatory submissions, and helps to implement new technology. Aust serves as a senior project manager for the Parenteral Drug Association. He earned his MBA from Ball State University and an M.S. degree from Purdue University in molecular and cellular biology.
Alexander Aust, M.S., MBA, is the co-founder and owner of Aust Business Solutions, a pharmaceutical consultancy based in Maryville, Tennessee, where he focuses on strategic planning and partnerships, and technical support. The firm provides CMC and project management services, assists with regulatory submissions, and helps to implement new technology. Aust serves as a senior project manager for the Parenteral Drug Association. He earned his MBA from Ball State University and an M.S. degree from Purdue University in molecular and cellular biology.
References
- https://www.trade.gov/faq/ftz-act
- https://www.cbp.gov/border-security/ports-entry/cargo-security/cargo-control/foreign-trade-zones/about
- https://www.fda.gov/industry/import-program-food-and-drug-administration-fda/fda-import-process
- FDA Guidance for Industry – cGMP for Phase 1 Investigational Drugs, July 2008
