Single-Use Platforms Accelerate Viral Vaccine Development And Manufacturing
By Amélie Boulais
Originally pubished in BioProcess International
Analyses of the biopharmaceutical industry predict that the market for vaccines could grow at a compound annual growth rate of as much as 10.3% between 2013 and 2024 (1). This is faster than growth predicted for recombinant proteins and monoclonal antibodies. Although traditional types of vaccines such as conjugated, inactivated, live attenuated, and toxoid vaccines dominate the market today, new generations of vaccines such as recombinant vector vaccines and subunit vaccine candidates dominate preclinical and clinical development phases. This implies that manufacturers are starting to commercialize a new generation of vaccines based on recombinant technology. Sartorius Stedim Biotech (SSB) is investing resources in understanding the future manufacturing needs of the vaccine industry. We are exploring the best ways of implementing single-use platforms for next-generation vaccines that avoid reinventing the wheel for each candidate, thereby reducing time to market, lowering production costs, lowering risks, and increasing flexibility.
In our experience, every vaccine’s process is distinct in one way or another. A vaccine product will have unique characteristics. The cell lines used in manufacturing will introduce their own nuances, and production processes will face individual safety considerations. For this reason, multiple platforms are needed to cover the different vaccine modalities. SSB has begun by developing a platform for viral vector applications. The platform’s predefined technologies can be integrated with one another to allow development and manufacturing of our customers’ vaccines. These technologies are complemented by unique services to support the vaccine company’s product across its entire life cycle.
An Upstream Toolbox for Vaccine Processing
Figure 1 shows the upstream processing technologies that viral vector manufacturers can use within their processes. The life-cycle approach is evident if we consider SSB’s portfolio of single-use bioreactors, which are readily scalable from the 250-mL ambr 250 high throughput bioreactor through to the 2,000-L BIOSTAT STR 2000 product. All of the vessels in the bioreactor range are geometrically similar to one another to ensure consistent mixing and gassing strategies during scale-up. This allows customers to simplify scale-up and scale-down studies, easily switch between conventional and single-use bioreactors, and reduce risk during process transfers.
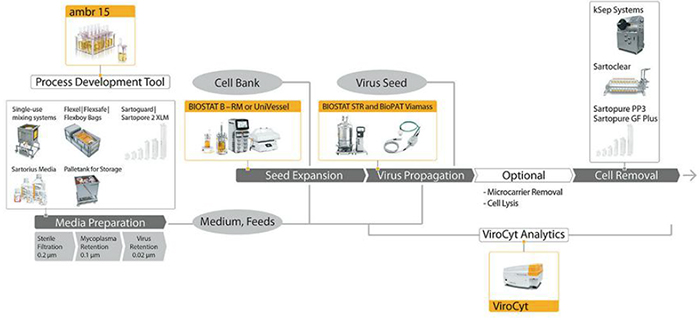
The ambr 250 high-throughput and modular systems are fully automated and allow large design-of-experiments (DoE) studies to be run quickly and easily during process development and optimization activities. To address development of adherent cell culture processes, SSB has developed a new generation of ambr vessels to support the growth of cells on microcarriers. SSB has recently published work describing the physical, computational f luid dynamics, and biological analysis of a microcarrier process run in the ambr 250 high throughput bioreactor (2). Vero cells were grown on microcarriers in an ambr 250 vessel with a modified design. During the next phase of development work, engineers will verify the new vessel design by culturing human mesenchymal stem cells (hMSCs) and will try to demonstrate the scalability of microcarrier cultures from the ambr 250 high-throughput through to SSB’s large-scale single-use bioreactors.
 Studies already performed with mammalian cells in suspension show that cell viability, cell concentration, and product concentration are highly reproducible from the ambr 250 modular unit to the 5-L BIOSTAT B, the 50-L BIOSTAT, and finally to the 1,000-L BIOSTAT bioreactors. Two vaccine manufacturer customers have verified the scalability of the SSB single-use bioreactor portfolio. Zoetis reported the successful scale-up of its upstream viral vaccine process from 2 L to the BIOSTAT STR 50 and then to the BIOSTAT STR 200 vessels. The company cultured BHK21 cells on microcarriers and found that the cell concentrations and virus titers were consistent between scales. Similarly, GSK described the successful scale-up of its BSL2+ culture from 10-L glass bioreactors to the single-use BIOSTAT STR 200 and then the BIOSTAT STR 1000 vessels. The single-use bioreactors gave equivalent performance to the 10-L glass bioreactor with regard to cell density and antigen titer.
Studies already performed with mammalian cells in suspension show that cell viability, cell concentration, and product concentration are highly reproducible from the ambr 250 modular unit to the 5-L BIOSTAT B, the 50-L BIOSTAT, and finally to the 1,000-L BIOSTAT bioreactors. Two vaccine manufacturer customers have verified the scalability of the SSB single-use bioreactor portfolio. Zoetis reported the successful scale-up of its upstream viral vaccine process from 2 L to the BIOSTAT STR 50 and then to the BIOSTAT STR 200 vessels. The company cultured BHK21 cells on microcarriers and found that the cell concentrations and virus titers were consistent between scales. Similarly, GSK described the successful scale-up of its BSL2+ culture from 10-L glass bioreactors to the single-use BIOSTAT STR 200 and then the BIOSTAT STR 1000 vessels. The single-use bioreactors gave equivalent performance to the 10-L glass bioreactor with regard to cell density and antigen titer.
Vaccine manufacturers often process live pathogens and, therefore, take safety considerations very seriously. By adding unique design features, SSB focuses heavily on ensuring that its bioreactors provide a high level of containment. It has adopted a systematic approach to preventing leaks from bioreactors by adapting a risk assessment that a vaccine manufacturing organization has published previously (3). Figure 2 summarizes the modified risk assessment (4).
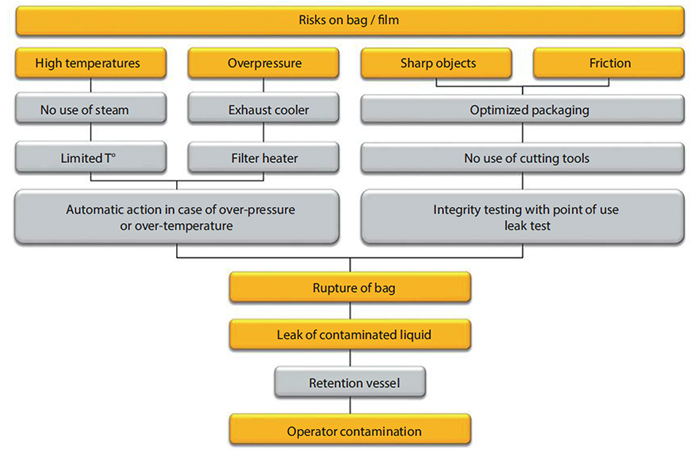
BIOSTAT STR bioreactors operate with bags constructed from the robust, multilayer Flexsafe film. SSB delivers the consumable portion in protective cleanroom-compliant packaging. Manufacturing operators can use a specifically designed installation device to place the 2,000-L bag into the holder. The bags are fitted with noninvasive sensors and with back-up lines for gas inlets and outlets. BIOSTAT STR bioreactors feature a containment tray that manufacturers can connect to a kill tank. This ensures that if a leak occurs, the contaminated material can be treated safely. The bioreactor automation includes numerous safety interlocks and shutdown mechanisms designed to protect the bags from excessive pressures and temperatures.
SSB has developed the Sartocheck bag tester for qualified pressure integrity testing of bioreactor bags. It detects leaks that may have been introduced before installation. The integrity test takes 24–60 minutes, including inflation and deflation, and tests the consumable up to the first clamp on the tubing. A fleece on the bag holder prevents defects from being masked by the bag holder itself.
We also have designed a single-use exhaust cooler to prevent filter blockages at high airf low rates. A blockage on the exhaust filter could lead to a pressure build-up that could damage the consumable and lead to a containment loss. Condensate is returned to the bioreactor bag to prevent product losses.
A Downstream Toolbox for Vaccine Processing
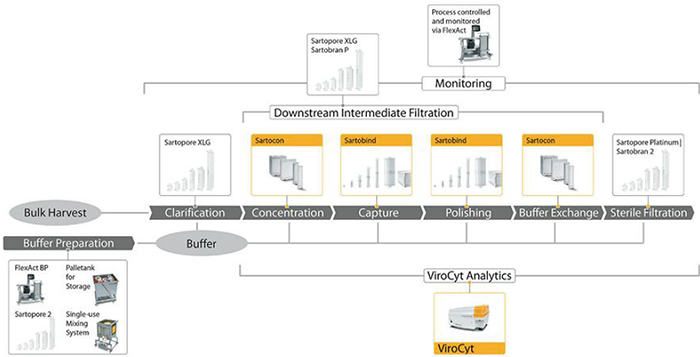
Figure 3 shows downstream processing technologies that viral vector manufacturers can use within their processes. SSB recommends that viral vector manufacturers use a membrane adsorber technology during viral vector purification. Membrane adsorbers have significantly larger pore sizes than conventional bead chromatography resins. This gives them more than tenfold greater binding capacity than columns for viral vaccines, virus-like particles, and large proteins. Vaccine producers also can operate membrane adsorbers at 10-fold higher flowrates. SSB has developed a unique cassette format to allow large-scale processing and provide higher flexibility. It will enable manufacturers of viral vectors to implement the technology during commercial production without encountering the scale limitation of 5-L membrane volumes - the largest size available in a classical capsule format. SSB design engineers have taken care to maintain the f low path principles throughout the range, ensuring that the membrane adsorbers are suitable for use at any stage in the lifecycle of a vaccine product.
Demonstrating the Platform Approach in Downstream Processing
SSB collaborated with IBET (Lisbon, Portugal) to develop a complete single-use downstream process for production of adenovirus (Ad5) with commercially available technologies (4). The unit operations used standard SSB technologies from the vaccine platform. The Ad5 vector was produced in HEK293 cells. Initial experiments were performed at the 2-L scale, and then the process transferred to the 20-L scale. Adenovirus was captured during downstream processing using a Sartobind Q anion-exchange membrane adsorber. Polishing was performed using the Sartobind STIC membrane adsorber. This is a salt-tolerant anion-exchange membrane, which was demonstrated to be highly effective for removing residual DNA impurities. The process delivered a product with a final host-cell protein concentration of less than 20 μg/mL, less than 10 ng/dose of host cell DNA, less than 5 ng/mL of benzonase, and a yield of 55%. The platform was operated “out-of-the-box” with little development work, and IBET scientists believe that the yield could be increased to 65% easily.
The case study shows how manufacturers can use the SSB viral vaccine toolbox to implement a completely single-use and scalable process for adenovirus purification. The use of membrane adsorbers allows for faster, simpler, and more economical processing. The large-scale run achieved recovery yields that were comparable with those reported in the literature and met product specifications and guidelines for preclinical and phase 1 studies.
New Toolboxes for Vaccine Development
The vaccine industry requires new tools to help bring its pipeline of new products to the market quickly, safely, and economically. Vaccines are too diverse a product category for a single platform approach to address all of the challenges. Instead, SSB has initially assembled a toolbox of technologies that are suitable for recombinant viral vector production and is gathering an increasingly large set of data to demonstrate the effectiveness of those technologies in viral vector processing applications. This toolbox includes consumables, systems, analytics, and automation. Further toolboxes will be developed to meet the needs of the vaccine industry. The company is commercializing breakthrough single-use technologies to meet the specific needs of vaccine producers. SSB vaccine platforms include associated services such as process development consultants, analytical testing, validation services, and application specialists that will accelerate product development and allow new vaccine concepts to become a reality.
References
- Joshi A. Vaccine Market Projected to Reach $77.5 Billion By 2024. PharmaTimes online, 16 April 2018; http://www.pharmatimes.com/web_exclusives/vaccine_market_projected_to_reach_$77.5_billion_by_2024_1232012.
- Rotondi MC, et al. Experimental and Computational Fluid Dynamics Studies of Adherent Cells on Microcarriers in an ambr® 250 Bioreactor. For further information please contact Royston-Info@sartorius.com.
- Ghislain Y, et al. Disposable Bioreactors for Viral Vaccine Production: Challenges and Opportunities. Biopharm Intl. (8, Supp.) 2010; www.biopharminternational.com/disposable-bioreactors-viral-vaccine-productionchallenges-and-opportunities.
- De Wilde D, et al. Biosafety Considerations for Single-Use Bioreactors. BioProcess Int. 16(9) 2018: i16‒i23.
- Boulais A, Hutchinson N, Linz F. Enabling Viral Vaccine Production: Implementing Process-Scale Adenovirus Purification with a Single-Use Platform. Genet. Eng. Biotechn. N. 36(20) 2016; https://www.genengnews.com/genarticles/enabling-viral-vaccine-production/5896.
Amelie Boulais is the Marketing Manager New Generation Vaccines, Vaccines Segment at Sartorius Stedim Biotech; amelie.boulais@sartorius-stedim.com
