Single-Cell RT-PCR of Laser Capture-Microdissected Purkinje Cells
Abstract:
RT-PCR is the most sensitive technique for the determination of gene expression, and many applications based on RT-PCR methodology exist such as quantitative and single cell RT-PCR. Laser capture microdissection (LCM) (1) is a new technology for the isolation of circumscribed cell groups or single cells in heterogeneous tissues like cerebellum. Therefore, in this study we combined LCM and RT-PCR for gene expression analysis of ß-actin and tyrosine hydroxylase in single Purkinje cells of rat cerebellar cortex using Promega reagents.
Introduction
Single-cell RT-PCR is the most sensitive technique for the determination of gene expression, but is often accompanied by problems such as template contamination and primer dimerization.
Laser capture microdissection provides precise extraction of cell groups down to the single cell level from tissue sections on uncovered slides (i.e., no coverslip) using thermoplastic polymer films activated by a laser beam. Section preparation and dissection can be performed within minutes, thus minimizing RNA degradation.
Purkinje cells are localized in the cerebellar cortex of mammals. These cells are responsible for many modulatory functions in the cerebellum. Using Promega AMV Reverse Transcriptase and Taq DNA Polymerase, we examined gene expression of the enzyme, tyrosine hydroxylase (TH), a marker of dopaminergic neurons and fibers, and ß-actin, a common housekeeping gene, in single laser-microdissected Purkinje cells of rat cerebellum.
Laser Capture Microdissection and Single-Cell RT-PCR
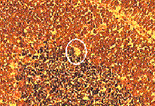
Cryosections of cryopreservated rat cerebellum (6µm) were prepared, mounted on uncoated slides at minus 30°C in a cryotome and stained with hematoxylin and eosin using a modified protocol (2). Laser microdissection was performed immediately. Single Purkinje cells were microdissected (Figure 1) with a PixCell-II using transfer films (MWG-Biotech, Ebersberg, Germany). Parameters for LCM included a 7.5µm laser spot size, 45mW power and 500ms duration. LCM transfer film containing the dissected cell was fitted into a 0.5ml microcentrifuge tube containing 12µl of lysis buffer (50mM TRIS-HCl [pH 8.0], 100mM NaCl, 5mM MgCl2, 0.5% Triton® X-100, 1mM DTT and 1000 units RNasin Ribonuclease Inhibitor, per milliliter buffer). This was followed immediately by centrifugation, freezing in liquid nitrogen and storage at –70°C.
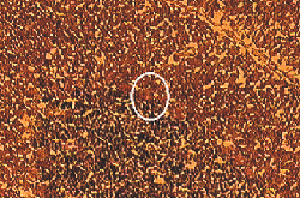

Laser capture micro-dissection of single Purkinje cell in rat cerebellum. Tissue before (Top Panel) and after (Bottom Panel) laser capture microdissection.
RT-PCR was performed according to standard protocols (3). The complete contents of the tubes (10µl) were reverse transcribed (AMV Reverse Transcriptase, 20 units) in the presence of both oligo(dT) and random primers. Next, cDNA was divided into 5µl for amplification of ßeta-actin and 15µl for TH using Taq DNA Polymerase. Amplification was performed using "hot start" with 2.5 units of Taq DNA Polymerase and the following parameters.
Cycling profile: initial denaturation of 94°C for 3 minutes, followed by 45 cycles of denaturation at 94°C for 1 minute, annealing at 58°C for 2 minutes and extension at 72°C for 2.5 minutes. a final extension at 72°C for 5 minutes was perfirmed and products were then cooled to 4°C.
Primers specific for -actin and TH spanned introns; expected product sizes from mRNA were 372bp for ß-actin and 301bp for TH. Aliquots of each sample were separated on a 2% agarose gel and visualized by ethidium bromide staining (Figure 2). The identity of PCR-products was confirmed by Southern hybridization and sequence analysis. Negative control reactions, containing all reagents except the reverse transcriptase or RNA, were performed in parallel. Total RNA of homogenized cerebellum served as positive control.
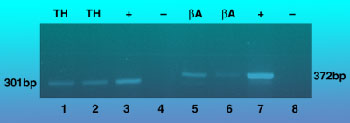
Representative 2% agarose gel showing PCR products following laser capture microdissection and single-cell RT-PCR. TH = tyrosine hydroxylase; ßA = ß actin; (+) = positive control; (--) = negative control. The negative control represented in lane 4 lacked RNA; the negative control represented in lane 8 lacked AMV RT. Postive control (lanes 3 and 7) included total brain RNA.
Conclusions
This study demonstrates the efficiency of the combination of laser capture microdissection and RT-PCR using Promega's AMV Reverse Transcriptase and Taq DNA Polymerase, as well as complementary reagents (e.g., dNTPs, 10X Reaction Buffer, oligo(dT) and random primers) for single-cell RT-PCR. These data also confirm previous findings that identified TH in cerebellar Purkinje cells by immunohistochemistry (4). Gene expression of TH in laser microdissected Purkinje cells is just one example of many applications for LCM/RT-PCR on single cells.
Acknowledgment
Peter Benoehr is supported by a grant of the Forschungsprogramm des Tübinger Universitätsklinikums (fortüne Nr. 408). The PixCell-II system was supported by a generous research fund of MWG-Biotech. We also thank Dr. Thomas Oehler, Promega GmbH, for his kind support.
References
- Simone, NL. et al. (1998) Laser-capture microdissection: opening the microscopic frontier to molecular analysis. Trends Genet. 14, 272.
- Modified staining protocol: Arcturus Engineering Inc.
- Revere Transcription protocol: For reverse transcription, the reaction included 40 units RNasin Ribonuclease Inhibitor, 5 units AMV Reverse Transriptase, 250ng oligo(dT)15 primer, 250ng random hexanucleotide primer, 10mM dNTPs, 2mM MgCl2, 10mM Tris-HCl, 50mM KCl and 0.1 % Triton® X-100. The reaction was kept at room temperature for ten minutes and then incubated at 42°C for 60 minutes. Denaturation was performed at 95°C for 5 minutes followed by immediate chilling on ice.
PCR protocol: For amplification, 50pmol of each specific primer, 2.5 units Taq DNA Polymerase, 10mM Tris-HCL, 50mM KCl, 0.1% Triton® X-100 and sterile water to a final volume of 50µl was used. - Takada, M., Sugimoto T. and Hattori, T. (1993) Tyrosine hydroxylase immunoreactivity in cerebellar Purkinje cells of the rat. Neurosci. Lett. 150, 61.
(a)The PCR process is covered by patents issued and applicable in certain countries. Promega does not encourage or support the unauthorized or unlicensed use of the PCR process. Use of this product is recommended for persons that either have a license to perform PCR or are not required to obtain a license.
This article is reprinted with permission from Promega eNotes.
