Sensitive Critical Starting Materials Need A 'Just-In-Data' Approach
By Victor Lien

Cell and gene therapies are redefining the boundaries of modern medicine, offering transformative outcomes for patients with previously untreatable conditions. Yet these therapies challenge nearly every convention of traditional pharmaceutical manufacturing.
Most significant among those challenges is the interface between viral vector production and cell therapy processing — a junction that remains the most critical source of technical and logistical risk in the cell and gene therapy (CGT) supply chain.
Unlike traditional biologics, where platform technologies and global supply chains are well established, CGTs operate in a dynamic landscape with evolving regulatory expectations, limited historical precedent, and high patient-specific variability. As commercial launches accelerate, the need for scalable, compliant, and digitally integrated platforms becomes overriding.
This article proposes a strategic blueprint for transforming the fragmented, high-risk interface between vector and cell therapy platforms into a unified, predictable, and compliant system. Grounded in regulatory principles and operational best practices, the framework reimagines the supply chain from reactive constraint to scalable capability.
Why Viral Vectors Can’t Be Stocked
Unlike monoclonal antibodies, which benefit from decades of platform maturity and long-term stability, viral vectors — such as lentivirus and adeno-associated virus — cannot be stockpiled. Their limited shelf life and stringent storage requirements, often involving ultra-low temperatures, make inventory buffering impractical.
Lentiviral vectors, commonly used in autologous CAR-T therapies, are particularly constrained. Their short stability profiles and high production costs necessitate a just-in-time manufacturing model.
Compounding this challenge is the regulatory classification of viral vectors as critical starting materials (CSM). Regulators require full traceability for each vector lot, including release data tied directly to the patient-specific cell therapy run.
Because vectors are classified as critical starting materials, they fall under heightened regulatory scrutiny. Sponsors must maintain direct oversight of release specifications, deviation investigations, and change control — even when manufacturing is outsourced.
The inability to stock vectors limits flexibility in scheduling and increases reliance on real-time data integrity. Any disruption in vector availability or quality risks derailing clinical schedules and compromising patient access.
Chronology Of Risk Transfer In Autologous Therapies
Autologous CAR-T therapies operate on a tight “vein-to-vein” timeline, where the patient’s own cells are collected, modified, and returned within days. Because vectors cannot be pre-stocked, their release data must align perfectly with cell processing initiation.
This creates a chronology of risk transfer:
- Vector manufacturing risk: Technical variability in vector production makes run failures a high-probability event. Without inventory buffers, such failures immediately impact clinical scheduling.
- Vector release risk: Lengthy quality control assays, particularly those measuring infectious titter, conflict with tight patient timelines. This often forces cell therapy facilities to begin processing before final vector release data is available.
- Cell processing risk: If the vector fails a critical quality attribute after cell processing has begun, the patient’s irreplaceable apheresis material may be lost.
This chronology creates a “risk stack” where upstream variability compounds downstream consequences. Without predictive analytics or real-time release strategies, sponsors are forced into reactive decision-making. A single vector deviation can cascade into patient delays, regulatory noncompliance, or product loss.
The Unified Platform Blueprint: From JIT To JID
To mitigate these risks, a unified platform must be established — one that enforces technical rigor and clear accountability across both vector and cell therapy operations.
The cornerstone of this platform is a shift from just-in-time to just-in-data, where data assurance precedes material movement. Much like air traffic control systems that validate flight paths before takeoff, just-in-data ensures that vector quality, release status, and scheduling alignment are confirmed before initiating cell therapy runs.
This shift supports enterprise-level governance, enabling sponsors to scale operations without compromising compliance or patient safety.
Harmonizing vector supply with cell therapy operations
In a multi-processes and multi-sites manufacturing environment, vector quality, timing, and data integrity must align with downstream cell therapy requirements. Four key challenges define the fault lines where operational risk concentrates:
- Release disconnect
- Logistical risk transfer
- Regulatory ambiguity
- Digital data silos
Each challenge demands a corresponding strategic imperative and technical integration. These are not stand-alone fixes — they form a tightly coupled system where failure in one domain can undermine the entire platform.
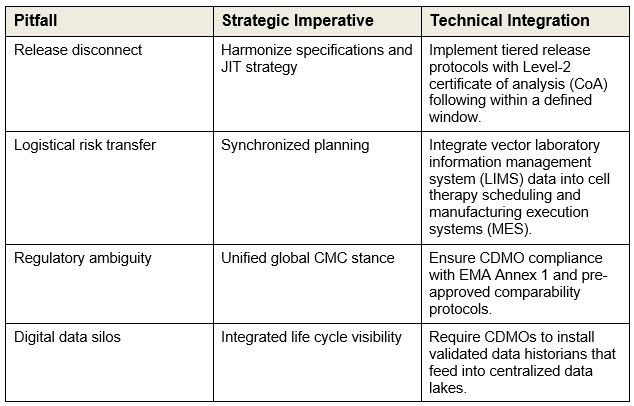
These imperatives form the foundation of a unified platform architecture. The following sections detail how each challenge is implemented through specific technical and governance mechanisms.
Tiered release protocols: resolving the release disconnect
The first and most immediate challenge — release disconnect — is addressed through a rigorously validated tiered release strategy. This system legally and scientifically divides vector release into two stages, enabling cell processing to begin with high-confidence data while longer assays are still underway.
- Level 1 (provisional release): Mitigates the analytical lag by confirming safety and identity through rapid assays, allowing cell therapy to proceed without waiting for full potency data.
- Level 2 (final release): Completes the compliance check with functional potency and extended safety assays before the final cell product is released to the patient.
Regulators increasingly support tiered release strategies when backed by robust risk assessments and comparability protocols. Sponsors must ensure that Level 1 assays are validated, rapid, and predictive of final potency outcomes.
MSAT oversight and technical validation: mitigating logistical risk transfer
To prevent scheduling a patient slot against a vector lot that may fail or be delayed, sponsors must enforce synchronized planning and embed technical assurance upstream. This is achieved through robust MSAT oversight and proactive process validation.
- Scale-down model (SDM): Enables MSAT-led deviation investigations and continuous improvement, ensuring that vector lots meet predefined quality thresholds before scheduling.
- Process analytical technology (PAT): Shifts assurance from end-of-process CoA to real-time monitoring, allowing early detection of deviations that could impact cell therapy outcomes.
MSAT teams serve as the technical bridge between CDMO operations and sponsor governance. These tools also support continuous improvement, feeding insights back into process design and regulatory filings.
Governance framework: addressing regulatory ambiguity and data silos
The final two challenges — regulatory ambiguity and digital data silos — require enterprise-level governance and digital infrastructure.
Unified global CMC strategy
To resolve regulatory ambiguity across global CDMO networks, sponsors need to pursue a “one-file” strategy:
- Comparability protocols (CPs): Ensure that vectors produced at different sites yield comparable cell therapy outcomes, enabling seamless tech transfer.
- Modular CTD submissions: Treat vector data as a standardized module, streamlining regulatory filings across geographies and products.
Governance frameworks must extend beyond compliance. They must enable strategic decision rights across global networks, including ownership of release decisions, deviation investigations, and change control.
Integrated life cycle visibility
To eliminate digital data silos and enable predictive control:
- Data historians and centralized data lakes: CDMOs must push high-fidelity process data into sponsor analytics platforms.
- MSAT dashboards: Advanced analytics link vector critical process parameters deviations to cell product outcomes, allowing early quarantine of suspect lots.
These tools support tech transfer by ensuring that process data from one site can be compared, validated and scaled at another — without compromising product quality or regulatory alignment.
Conclusion
The incapability to stock viral vectors elevates their role from a supply component to a strategic interface. By resolving the four core challenges — release disconnect, logistical risk transfer, regulatory ambiguity, and digital data silos — sponsors can transform a fragmented supply chain into a scalable capability.
Platform thinking transforms fragmented operations into scalable capabilities. By integrating governance, digital infrastructure, and technical rigor, sponsors can shift from reactive troubleshooting to predictive control.
This not only improves operational resilience, it ensures that every patient receives a safe, timely, and effective therapy. When every moment counts and delays have serious consequences on patients, execution with care and discipline isn’t just business necessity – it’s the right thing to do.
About The Author:
 Victor Lien, Ph.D., is a CMC strategy and platform developer with extensive experience across several advanced modalities, including antibody-drug conjugates, cell and gene therapies, and mRNA. He most recently worked as a director of biotherapeutic pharmaceutical sciences at Pfizer. Before that, he was a director of CMC and tech transfer at Gracell Biotechnologies, now a part of AstraZeneca. Other experience includes leadership positions at Bristol Myers Squibb, DowDuPont, Fluidigm, and Vertex Pharmaceuticals. He was also a systems biology instructor at Harvard Medical School. He received his Ph.D. from the University of California San Diego.
Victor Lien, Ph.D., is a CMC strategy and platform developer with extensive experience across several advanced modalities, including antibody-drug conjugates, cell and gene therapies, and mRNA. He most recently worked as a director of biotherapeutic pharmaceutical sciences at Pfizer. Before that, he was a director of CMC and tech transfer at Gracell Biotechnologies, now a part of AstraZeneca. Other experience includes leadership positions at Bristol Myers Squibb, DowDuPont, Fluidigm, and Vertex Pharmaceuticals. He was also a systems biology instructor at Harvard Medical School. He received his Ph.D. from the University of California San Diego.
