Rethinking Aseptic Filling: Innovations To Meet Pharma's Challenging Requirements
Current trends in drug development point toward a growing number of therapies that are targeting smaller patient populations. As the batch sizes required to manufacture clinical and commercial supply of these therapies are smaller than in the days of blockbuster medicines, the equipment and processes for manufacturing have had to change as well, creating challenges for smaller pharmaceutical companies.
A major factor of decreasing batch size is the steady increase of therapies for rare diseases. This trend has continued in 2019, where the number of drugs in development increased over 2018 by seven percent to almost 5,000 drugs (PharmaProjects, January 2019). Another reason for smaller batch size is the targeting of existing drugs to more specific patient populations. Examples of this are Roche’s Herceptin® and Kadcyla® products. Herceptin was originally developed for a specific subset of breast cancer patients. Kadcyla incorporates the Herceptin antibody but is an even more targeted therapeutic, servicing an even smaller subset of patients with breast cancer.
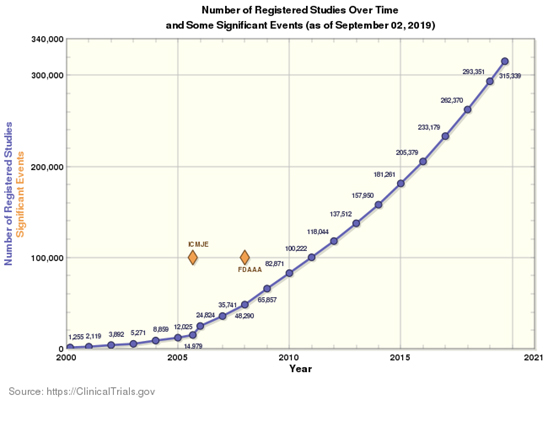
As seen by the graph from the U.S. National Library of Medicine, there has been a steady increase in the number of drugs in phase 1 and 2 clinical trials, resulting in more demand for clinical-stage manufacturing. Of the studies launched in 2018, 46% were approved based on data from trials with fewer than 500 subjects in total.
Smaller Batch Sizes Create Manufacturing Challenges
The smaller patient populations require less product to be developed and manufactured. This trend has created a need to produce a larger mix of products with increasing complexity, often in different container formats, in small volumes, with limited and expensive drug product. These requirements become problematic for both the pharmaceutical companies and the Contract Manufacturing Organizations (CMOs) that these pharmaceutical companies rely on. From a CMO perspective, the challenge in meeting these requirements is compounded by the constant push to get products through clinical development and to market faster. Anything that can reduce lead-times has a profoundly significant impact on both the developer and the patient population it serves. Typical large CMOs have validated equipment and processes in place to efficiently support large batch sizes and repetitive manufacturing of commercial products; therefore, they prefer large volume product manufacturing opportunities. The requirements of clients needing just a small number of units filled for an early phase study do not fit into that paradigm. A large conventional CMO may turn away Phase 1 customers because they may not have technology for small batch filling, and the customer may look elsewhere when faced with significant manufacturing line losses. When you only have a few liters of a very expensive experimental drug product, having 1 L of line loss is too high a price to pay.
Being a virtual or small pharma company has its benefits, but the need to outsource all services including manufacturing often means taking a back seat to the higher volume, higher paying customers that command a CMO’s capacity. This can lead a more specialized pharma company, or even large pharma with specialized programs, to look for alternatives to the high volume CMOs. When taking this route, pharmaceutical companies run the risk of ending up with smaller service organizations that lack the state-of-the-art equipment and support infrastructure the large CMOs have. The more manual a process, the more opportunity for variations, deviations, and contamination, which could expose the drug product and company to regulatory and quality issues. Smaller service organizations with the capabilities to do small, specialized batch sizes with a less manual approach can result in fewer deviations and a better overall success rate with fills.
Leveraging Technology Advances to Meet the Challenge
There have been technological advancements in aseptic filling implemented over the years aimed at closing these capability gaps. One of the first initiatives meant to address the issues with manual filling was isolator technology. Isolator technology was developed to reduce risk by surrounding the process with barriers and isolating the operator from the environment. This allowed the installation of filling lines in more-economical, lower-grade cleanrooms.
Even though isolation technology was on the right track, a complete solution had still not been realized. Isolation was able to remove people from the environment but was unable to remove people from the process. Gloves were, and continue to remain, a source of contamination. Quite simply, where there are gloves, there is an opportunity for operators to interfere in the process. The large isolators also take a long time to set-up, decontaminate, and switch over to different formats. These long set up times encourage large batches and campaigning products in the same format, which does not meet the emerging need for flexible filling capability.
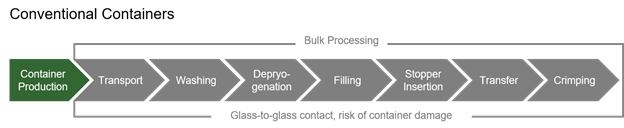
Other innovations, such as pre-sterilized components, have helped as well. Pre-fillable syringes (PFS) in nested tubs have been available for the past two decades and have become an industry standard. PFSs reduce in-house processes and equipment needs and simplifies the supply chain (see graphic below). In addition, the higher price of nested PFSs has been offset by the cost savings of reduced capital equipment and lower operating costs.
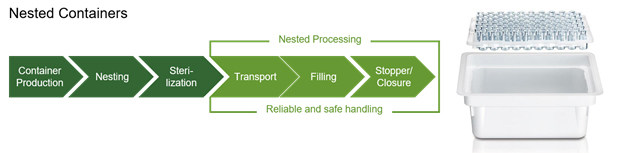
Taking it a step further, ready-to-use (RTU) vials and cartridges are now being offered in nested formats, as well as closures. There is also an increasing availability of filling machines utilizing the nested formats.
The simplicity with conveying nested components makes the filling process much more reliable. While the cost and lower density of nested RTU components does not lend itself to the large batch approach, it solves many of the challenges for the small-quantity batches that are becoming more common.

Outsourcing the prep/washing/sterilization/depyrogenation to qualified vendors who specialize in those operations allow the CMO to focus on their strengths. The nested format also makes batch traceability and segregation simpler.

Singota Solutions implemented one of the latest advancements in filling technology systems, the Vanrx SA25 Aseptic Filling Workcell. This unit takes advantage of the RTU component formats and utilizes a more efficient isolator process. The use of pre-sterilized components in standardized tubs has enabled the use of new material handling approaches. The SA25 is an innovative manufacturing workcell that utilizes robotics for fully automated component handling, filling, stoppering, and capping integrated within an isolator.

Why Singota Solutions Adopted Advanced Technology
The SA25 state-of-the-art technology offers many advantages in meeting the challenges Singota’s customers face with small batch filling. The nested RTU formats simplify and speed-up product changeovers. The system eliminates the interfaces between the isolator and the background environment for adding components and transferring vials downstream to a capping operation. Changeover tooling is simply a few material handling fixtures for the various tubs and formats.
A major factor in Singota’s choice of the SA25 was the elimination of glove ports. This design feature provides major benefits including eliminating the human element in the filling process, which reduces the opportunity for error. It also allows for rapid aeration times to reduce residual Hydrogen peroxide.
Pre-sterilized single-use fill-path components are disposed of after each run, eliminating cleaning validation of product contact parts. There is only one nest of containers exposed to the ISO 5 environment inside the filling isolator at a time. This limits the exposure to the drug product during the manufacturing process. All these advantages play into a system well suited to address the flexibility needed in the small volume therapeutics market.
Flexibility Cannot Sacrifice Quality
Several mechanisms of control are placed within the SA25. Recipe parameters and batch data are accessible through a single Human-Machine Interface (HMI), allowing for unified data records. Machine vision verifies incoming containers and completion of critical tasks. Pump parameters are part of the same recipe that drives batch production with the plunging needle and fill velocity and are all controlled by the specific recipe for each fill. Non-destructive In-Process Checks (IPC), frequency, and pump calibration processes are set as well. A precision peristaltic pump delivery system ensures tight fill tolerances and helps to minimize shear, which is especially important for sensitive biologics.
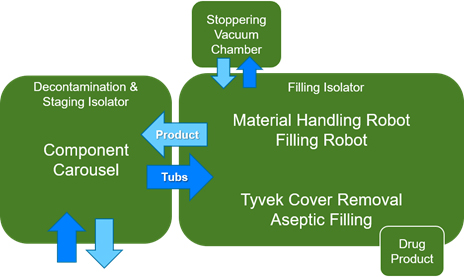
Quality improvements have been made in environmental monitoring as well. Conventional filling systems are much larger, more complex, and must address operator interventions. Gloves and complex systems result in higher particle loads which require multiple sampling locations for both viable and non-viable particles. Traditionally, the act of observation or measurement can have an impact on the quality being measured. Every viable sample is a risk of a false positive. There can also be a risk of introducing contamination into an aseptic environment.
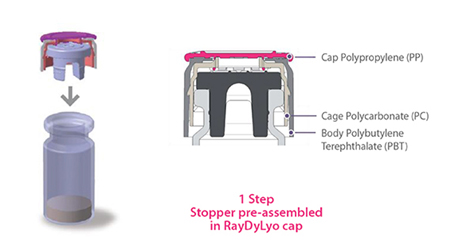
Another important feature is the use of a press-fit vial closure system when using vials as drug product containers. This press-fit vial closure system allows the vials to be fully sealed within the ISO 5 environment and eliminates the particulate generated from typical aluminum crimping systems. These press-fit caps have been proven to pass all forms of CCIT.
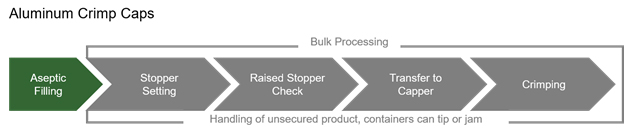
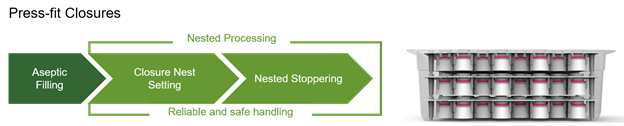
Press-fit vial closures, combined with the RTU nesting format, improves quality levels of the products manufactured. In addition to eliminating the need for crimping of aluminum caps, vibratory stopper bowls and tracks that are notorious sources of particulates, are eliminated from use in the isolator.
A Changing Service Model to Address Market Demand
Singota Solutions was facing the same market challenges other CMOs are facing. As said before, these include:
- Larger variety of products
- Increased complexity
- Different container formats
- Small batch sizes
- Limited and expensive drug materials
- Increased time-to-market pressure
Singota wanted to focus on better serving its clients by providing the capability to handle niche therapeutics with improved quality, speed, and flexibility. The added innovations and advancements in aseptic manufacturing filling technology adopted by Singota helped in meeting these needs. Just as important, however, was updating its service model to complement the technical innovations.
An important part of the company mission at Singota has always been to help move products through the development cycle faster. Lead-times are important to clients, so they are important to every employee. Fast, transparent, and responsive service to all clients, big or small, is part of the organizational culture. Methods and tools that promote and enable close and effective working relationships towards common goals among various functions within the organization are critical. Daily stand up meetings, frequent face-to-face communications, accountability for follow up of tasks, and coordination of ever-changing schedules are vitally important.
Next, to reduce lead times, Singota tackled the issue of long lead times for containers (syringes, vials, and cartridges). Singota’s approach was to identify and keep in stock commonly used containers and parts. Several sizes and styles of vials and stoppers can satisfy the majority of the market need. By focusing on an initial few that will cover a decent swath of user requirements, a project can start moving through the process more quickly.
Another element in the service model Singota adopted is having key services and capabilities to support client needs. Formulation development is important for projects in early development stages. The capability to provide stability studies is critical to evaluate product during various development phases. Finishing services geared to handle small and diverse runs is additionally helpful for clients filling small batches under strict timelines. Singota’s supply chain management service can handle APIs, excipients, drug products, and distribution to clinical sites, rounding out Singota’s services.
Speed to Market Through Technology and Service
Singota has put this technology and service model to the test with a recent client. After receiving positive results from an early Phase I trial, a new client for Singota was able to take advantage of the standard components in Singota’s inventory, the flexible filling capability, and streamlined collaboration. Within three weeks after signing a project proposal, the client was on-site filling an engineering batch and a subsequent GMP batch for the trial the following month.
Overall, the changing market requires innovative technologies along with a willingness to adapt business models to serve pharmaceutical companies who need high quality, cost-effective, small batch solutions. Rethinking aseptic filling from technology to process to service while meeting shifting market demands will help all participants in the pharmaceutical industry, and ultimately help the smaller patient populations who need targeted therapies.
