Research Explores Alternative To EU-Banned Triton X-100
By Onur Arslan, Robert Weik, and Philipp Mundsperger, Polymun Scientific; Sam Pallerla, Daniel Craig, Ryan Swoyer, and Tracy Blumen, IAVI
Pharmaceutical products derived from mammalian cells hold a risk of viral contamination. Viruses are adventitious agents that may be carried through to the product despite GMP environmental controls. They can be introduced by the master cell bank but also at various time points throughout the production.
Because of this risk, every process needs to include two orthogonal steps of viral inactivation/removal (detergent treatment or low pH incubation) and virus removal (chromatography or filtration), according to ICH Q5A(R2). These measures are essential to keep biopharmaceuticals safe.
To determine the ability of a production process to inactivate and remove viruses properly, virus clearance studies are conducted with model viruses. Mice minute virus (MMV, a non-enveloped virus) and murine leukemia virus (X-MuLV, an enveloped virus) are two prominent candidates for such studies that have been widely used. Solvent/detergent treatment or just detergent treatment works only on enveloped viruses. The solvent/detergent mixture, or just the detergent, disrupts the lipid membrane of enveloped viruses. Once disrupted, the virus is rendered inactive and can no longer bind to cells and infect them. This treatment is ideal for products that cannot tolerate low pH conditions, which is one of the alternative virus inactivation strategies.
One of the best-known detergents traditionally used is Triton X-100, which is no longer authorized for use in the European Economic Area. Because degradation products of Triton X-100 have detrimental impact on the environment1, the European Chemicals Agency, through the regulation No. 1907/2006 “Registration, Evaluation, Authorization, and Restriction of Chemicals” (REACH), identified Triton X-100 as a substance of very high concern on Dec. 19, 2012. The date from which the use of Triton X-100 was prohibited, unless an exemption is granted, was Jan. 4, 2019 and is now included in Annex XIV of REACH ("Authorization List").
Therefore, many pharmaceutical companies wanting to manufacture cell culture-derived biopharmaceuticals within the EU, now or in the future, face the challenge to replace Triton X-100 with a more environmentally friendly detergent. Naturally, the new detergent must fulfill the original purpose of virus inactivation with the same effectiveness as Triton X-100. One promising candidate is Virodex TXR-1, which was tested during the production of a nanoparticle-based HIV immunogen.
Two important aspects were investigated as part of this work: first, that the inactivation is robust and effective, i.e., rapid and complete, and second, that the process and product are not negatively influenced by the detergent.
Material And Methods
Material
Virodex TXR-1
Monograph names: macrogol lauryl ether 9 (Ph. Eur.), polyoxyl 9 lauryl ether (USP). Virodex TXR-1 is a readily biodegradable, non-ionic surfactant for viral inactivation and cell lysis, produced by Croda.2 The material is the key ingredient of Polidocanol, an FDA-approved injectable drug for the treatment of small varicose veins.3
The material is in compliance with USP and Ph. Eur. monographs.
Process intermediate
Test virus
Murine leukemia virus (X-MuLV; family retroviridae) is an enveloped, midsize (~70 nm to 100 nm), single-stranded RNA virus (pNFS Th-1 Xenotropic strain: ATCC VR-1447) with a low resistance to physio-chemical inactivation. This class of retrovirus (C-type) is present as an endogenous element in murine hybridomas and in Chinese hamster ovary (CHO) cells. Therefore, X-MuLV is used as a representative C-type retrovirus. It is obligatory to demonstrate inactivation and clearance of this class of virus by downstream processing steps used for the purification of biological products derived from monoclonal and CHO derived recombinant products.
Method
Manufacturing process
Upon storage at room temperature, the detergent can enter a solid phase. In order to liquify it, it must be heated to 40 degrees C before use. One option is to use a water bath at 40 degrees C and incubate the Virodex TXR-1 container for approximately 10 minutes until it is fully dissolved. The detergent is then added to the virus inactivation start material at a final concentration of 0.1 % and stirred for at least 10 minutes until it is completely dissolved. The product is then incubated for 1 to 2 hours at room temperature (18 to 26 degrees C) to ensure that the virus inactivation is successful.
Viral clearance study
Method description
An aliquot of the starting material is placed into a jacketed beaker and equilibrated to 17 ± 1 degrees C while being continuously stirred. The material is then spiked with the X-MuLV at a ratio of 10%. To perform the incubation, detergent is added to achieve a final concentration of 0.09%. As the final concentration used in the viral clearance study is below the actual final concentration used in a production run (0.1%) this concentration represents the worst-case scenario. The inactivation was performed at 17±1 degrees C for 60 minutes, with samples taken at various time points and immediately diluted in medium and treated with C18 resin to remove the detergent (see below).
Analytical methods
TCID50 assay
Samples are titrated by preparing serial 0.5 log10 dilutions of each sample. One hundred µL of each dilution are then inoculated in eight-fold replicates onto 96-well tissue culture plates seeded with the respective cell line. Following incubation at 36 ± 2 degrees C, 5% ± 1% CO2, 90% ± 5% humidity for 7 ± 1 days for X-MuLV, cells are evaluated for evidence of a cytopathic effect originating from the virus. Virus titers were calculated using the Spearman-Kärber method.4
For the final product sample where a low titer is expected, in addition to the method above, a bulk titration is performed using a larger volume at the first dilution (≥ 10 mL) to increase the sensitivity of the assay. A negative control plate, inoculated with the appropriate cell culture media, and a positive control plate, inoculated with the spike virus for which the titer was determined and checked to be within the defined range titer, ensured the validity of each set of titrations.
Cytotoxicity testing for TCID50 assay
This test is used to determine the dilution at which it is not cytotoxic to the matrix of the samples tested in this study and does not have any impact on the health status of the cells (i.e., cell death or morphological changes). Each sample is pH checked and if necessary adjusted to pH 6-8 with either NaOH or HCl solution and is then 0.45 µm filtered.
Starting material without detergent was 1 log10 pre-diluted in medium for virus titration prior to cytotoxicity testing. Starting material with detergent was 0.5 log10 diluted with citrate supplemented medium (pH 6.0 ± 0.2) and C18 resin added to reduce the concentration of free detergent. After mixing and centrifugation at 1,000 g for one minute, the supernatant was 0.5 log10 diluted, 0.45 µm filtered and titrated.
Serial 0.5 log10 dilutions are prepared in the respective medium for virus titration. One hundred µL of each dilution is then inoculated in eight-fold replicates onto 96-well tissue culture plates seeded with the respective cells. Following incubation cells are evaluated for evidence of any cytotoxic effects that might interfere with the interpretation of the virus titration assay.
Interference testing for TCID50 assay
Interference testing is performed to investigate if the matrix of the samples interferes with the ability to read the assay or if the product matrix itself results in virus inactivation. Each sample is pH checked and adjusted, if necessary, to pH 6-8 with NaOH or HCl solution prior to spiking.
Starting material with detergent was 0.5 log10 diluted with citrate supplemented medium (pH 6.0 ± 0.2) and C18 resin added to bind free detergent. Two aliquots of the resulting suspension are spiked in a ratio 1:100 with pre-diluted (in appropriate cold cell culture medium) virus stock suspension to a calculated titer of 3.5 log10 [TCID50/mL] and 2.5 log10 [TCID50/mL]. After mixing and centrifugation at 1,000 g for one minute, the supernatant is 0.5 log10 diluted with medium for virus titration, mixed immediately, 0.45 µm filtered and titrated.
As a control, an aliquot of the respective cold cell culture medium (PG-4 medium for PG-4 cells) is spiked in a similar manner with pre-diluted virus stock suspension before a sample is drawn for titration.
Following incubation cells are evaluated for evidence of any effects that might interfere with the interpretation of the TCID50 assay.
Enzyme-Linked Immunosorbent Assay (ELISA)
The quantitative determination of the nanoparticle-based HIV immunogen is carried out using ELISA. A specific antibody to the immunogen is used to coat the ELISA plate. In the first reaction step, the protein nanoparticle binds to the antibody that is adsorbed on the inner surface of the well. In the second reaction step, a specific biotinylated antibody binds to the already bound target protein. In the third reaction step, the protein conjugated with horseradish peroxidase reacts with the bound detection antibody. The enzyme activity of the peroxidase oxidizes the colorless, chromogenic substrate to an orange-colored product.
After reaching a sufficient absorbance, the reaction is stopped by denaturation of the enzyme with a stop solution (H2SO4). The intensity of the color correlates with the amount of bound nanoparticle-based HIV immunogen.
Results
Virodex TXR-1 was chosen for process optimization because its chemistry conforms to Ph. Eur. and USP-NF standards. It is listed as macrogol lauryl ether 9 (Ph. Eur.) and polyoxyl 9 lauryl ether (USP). The surfactant was tested for handling and interaction with the target protein and process. Its handling is similar to Triton X-100, although it may need to be heated to 40 degrees C, such as in a water bath, to dissolve the solid phase.
Once dissolved, pipetting the surfactant is straightforward, especially for operators experienced with other detergents. Virodex TXR-1 had no negative impact on the process and product. The detergent inactivation step yields were excellent, as shown in Table 1.
In addition, the ability of the downstream sequence to remove the detergent throughout the process was tested. An RP-HPLC was used to analyze the amount of detergent in every product intermediate. Results in Table 2 reveal that after the first anion exchange step, the detergent is almost completely removed (2 ppm) and after the next purification step (HAC) the amount of Virodex TXR-1 is below the limit of detection. The fact that the detergent is completely removable is another reason that it can be considered a good option to replace Triton X-100.
Table 1: Viral inactivation results
| Process parameter | L17 | L19 | L20 | L21 |
|---|---|---|---|---|
| Step yield (ELISA) [%] | 100.1 | 106.6 | 86.5 | 108.4 |
Table 2: Removal of Virodex TXR-1
| Process step | Expected amount [ppm] | Actual amount [ppm] |
|---|---|---|
| Detergent inactivation | 1000 | 998 |
| AEX 1 eluate | 0 - 5 | 2 |
| HAC eluate | 0 - 2 | -1) |
| MM flow through | 0 - 2 | -1) |
| CEX eluate | 0 - 2 | -1) |
| AEX 2 eluate | 0 - 2 | -1) |
| UF/DF | 0 - 2 | -1) |
| NF | 0 - 2 | -1) |
1) Either below buffer blank or not detectable, therefore virtually no detection.
Virus Clearance Study
The study was conducted in collaboration with ViruSure GmbH, Vienna, Austria, at their facilities. The goal was to determine the viral clearance of this process step and to provide more information about the cytotoxicity of Virodex TXR-1.
Cytotoxicity testing revealed that the first non-cytotoxic dilution is 1.5 log for the start material without detergent (2.5 log overall, considering the 1.0 log predilution) and 1.0 log (2.0 log overall) for the starting material with detergent (actual process start material) after C18 resin treatment. Initial tests showed that the cytotoxicity in samples containing Virodex TXR-1 was too high for analysis with the TCID50 assay.
Therefore, C18 treatment was introduced to remove the detergent. After completing cytotoxicity and interference testing, the actual viral clearance was investigated. The study showed that the detergent treatment step rapidly and completely inactivated X-MuLV and it can be considered an effective step for the inactivation of enveloped viruses. See Table 3 and Figure 1.
Table 3: Summary of virus reduction factors
| Reduction factors [log10] ± 95% Cl | X-MuLV | |
| Run 1 | Run 2 | |
| SSM vs Hold | 0.13 ± 0.44 |
- 0.13 ± 0.48 |
| SSM vs Kinetics point t=0 | ≥ 2.24 ± 0.31 |
≥ 2.37 ± 0.34 |
| SSM vs Kinetics point t=5 | ≥ 2.24 ± 0.31 |
≥ 2.37 ± 0.34 |
| SSM vs Kinetics point t=15 | ≥ 2.24 ± 0.31 |
≥ 2.37 ± 0.34 |
| SSM vs Kinetics point t=30 | ≥ 2.24 ± 0.31 |
≥ 2.37 ± 0.34 |
| SSM vs Kinetics point t=60 | ≥ 4.67 ± 0.32 |
≥ 4.79 ± 0.34 |
Figure 1: Inactivation kinetics of X-MuLV
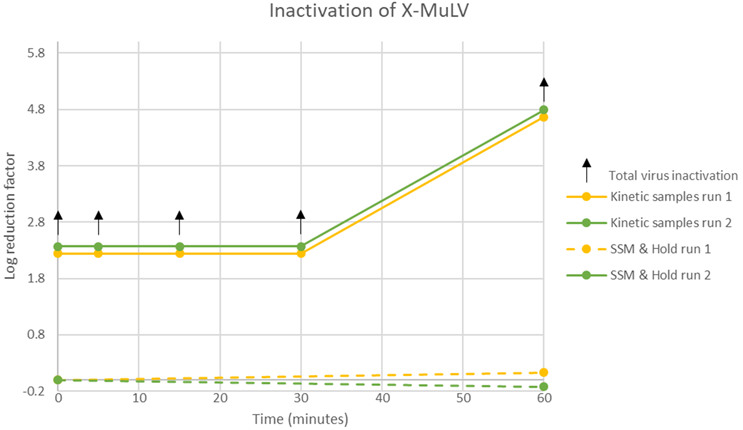
It is important to understand that the T-0 time points (SSM & Hold Run 1 and SSM & Hold Run 2) show complete inactivation and therefore inactivation does not improve after 60 minutes. For the two T=60 kinetic samples, a higher volume was titrated in order to improve the LOD (limit of detection) of the assay and therefore yielded a higher reduction factor.
Conclusion
The European Chemicals Agency has included Triton X-100 and related molecules on the REACH Annex 14 regulation, forcing the European pharmaceutical industry to find alternatives. One such alternative is Virodex TXR-1. The good viral clearance information provided by the manufacturer (Croda) and the fact that it is Ph. Eur. and USP compliant made it the detergent of choice to test as a replacement for Triton X-100. After several process optimization runs, one large-scale GMP run, and a virus clearance study, Virodex TXR-1 can be considered a good replacement for the recently banned Triton X-100. No negative effects on the detergent step yield or overall downstream process could be observed, while also achieving rapid and complete viral clearance. In addition, it has been shown that the detergent is removed to below the limit of detection after two chromatography steps in the downstream process.
If the step directly after the detergent treatment is also part of the viral clearance, the high cytotoxicity of Virodex TXR-1 needs to be addressed. In this scenario, the detergent is present in the starting material of the viral clearance study of the subsequent step. The cytotoxicity affects the spiked virus and makes analysis with the TCID50 assay challenging. This problem also occurred in the viral clearance study of the nanoparticle-based HIV immunogen. In order to determine the virus removal, the TCID50 assay was successfully replaced by a qPCR assay for the subsequent downstream step. qPCR does not distinguish between active and inactive viruses and is not influenced by the detergent. Therefore, this assay is better suited to determine the removal of viruses.
Acknowledgements: This project was funded in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. 75N93022D00005.
Authors’ note: Special thanks are also due to Andy Bailey and Konstantinos Papakostas from ViruSure GmbH. Their support in conducting the viral clearance study and extensive knowledge were a great benefit to this article.
References:
- White R, Jobling S, Hoare SA, Sumpter JP, Parker MG. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994 Jul;135(1):175-82. doi: 10.1210/endo.135.1.8013351. PMID: 8013351.
- Hunter AK, Rezvani K, Aspelund MT, Xi G, Gadre D, Linke T, Cai K, Mulagapati SHR, Witkos T. Identification of compendial nonionic detergents for the replacement of Triton X-100 in bioprocessing. Biotechnol Prog. 2022 Mar;38(2):e3235. doi: 10.1002/btpr.3235. Epub 2022 Jan 22. PMID: 35043591; PMCID: PMC9285696.
- National Center for Biotechnology Information. PubChem Compound Summary for CID 656641, Polidocanol. https://pubchem.ncbi.nlm.nih.gov/compound/Polidocanol. Accessed Aug. 26, 2024.
- Ramakrishnan MA. Determination of 50% endpoint titer using a simple formula. World J Virol. 2016 May 12;5(2):85-6. doi: 10.5501/wjv.v5.i2.85. PMID: 27175354; PMCID: PMC4861875.
About The Authors:
 Onur Arslan, M.Sc., is deputy head of downstream processing at Polymun. Onur Arslan, M.Sc., is deputy head of downstream processing at Polymun. |
 Robert Weik, Ph.D., is the head of downstream processing at Polymun. Robert Weik, Ph.D., is the head of downstream processing at Polymun. |
 Philipp Mundsperger, M.Sc., is a project manager at Polymun. Philipp Mundsperger, M.Sc., is a project manager at Polymun. |
 Tracy Blumen, M.S.Ch.E., is senior director of portfolio management at IAVI. Tracy Blumen, M.S.Ch.E., is senior director of portfolio management at IAVI. |
 Sam Pallerla, Ph.D., PE, is senior director of product development and manufacturing at IAVI. Sam Pallerla, Ph.D., PE, is senior director of product development and manufacturing at IAVI. |
 Ryan Swoyer, M.Sc., is the director of analytical science and assay development at IAVI. Ryan Swoyer, M.Sc., is the director of analytical science and assay development at IAVI. |
 Daniel Craig, Ph.D., is associate director of CMC recombinant protein development at IAVI. Daniel Craig, Ph.D., is associate director of CMC recombinant protein development at IAVI. |
