Reducing Upstream Processing Scale-Up Risks Using QbD

By Mario Becker, Tim Ward and Christel Fenge
Scientists and engineers seeking to develop manufacturing processes for new biopharmaceutical drugs face a number of challenges. Upstream process development must deliver a high-yielding cell culture to meet Cost of Goods (CoGs) objectives. The process must be robust to ensure high batch success rates with a low risk of contaminations and minimal variations in cell growth performance. The biological product must maintain the required product quality attributes, during scale-up from laboratory to commercial scale. Biopharmaceutical companies must address these complex challenges in the shortest possible timeframe and at reasonable effort and cost during development. Doing so allows the early evaluation of products in the clinic and, therefore, effective resource allocation behind projects from the pipeline that are most likely to be successful all the way to the market.
Adopting a platform approach to cell line development and using automated high-throughput miniaturized bioreactors allows companies to increase the speed with which they bring their products to the clinic. Complementing these techniques with a carefully considered approach to cell culture scale-up ensures right-first-time manufacturing. The objective during scale-up is to transfer seamlessly the process from the laboratory through clinical trial manufacturing, process characterization and then commercial manufacturing. Doing so requires a high level of process understanding that leads to greater predictability, flexibility and the avoidance of surprises when operating at the large-scale.
Bioreactor vessel design to facilitate process scale-up
The ease with which processes transition between production scales increases when the bioreactors used at each scale are geometrically similar. Companies, such as Merck Research Laboratories, have successfully and efficiently optimized cell culture processes at the 250-mL scale using design of experiments (DOE) methodologies and the ambr250® microbioreactor workstation (Bareither et al., 2015). The vessels for the ambr250® have the classical stirred tank bioreactor design with a height to diameter ratio of approximately two and an impeller diameter to vessel diameter of 0.4. These ratios are consistent with those of 2-L UniVessel® SU and BIOSTAT STR® single-use bioreactors allowing straightforward scaling from 250mL to 2000L (Figure 1).
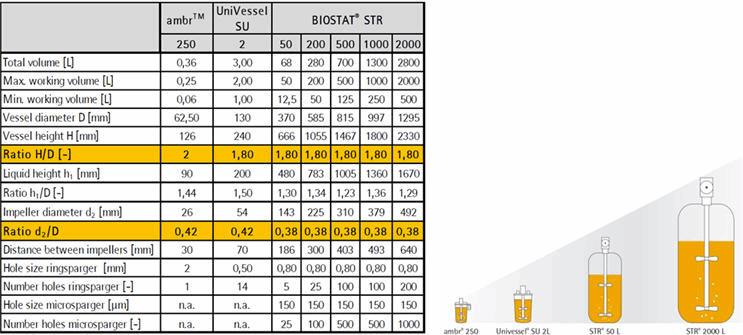
Figure 1: Straightforward bioreactor scalability with a classical stirred tank design
Scale conversion tools allow the translation of scale-dependent parameters such as agitation speed or gas flow between different scales of bioreactors based on choosing a preferred scaling principle, e.g. power input, tip speed, Kolmogorov eddy length or kLa. Using such tools enables engineers to understand the relevant operating window of their small-scale process development models so that the data they generate are representative of their large-scale production bioreactors (Namdev, 2006). Based on this knowledge, engineers can then define the design space of their cell culture process in their small-scale model that is representative of their large-scale production bioreactors (Figure 2). Failure to do so would risk the development of cell culture processes that are inoperable once scaled-up.

Figure 2: Optimal DoE planning according to known bioreactor operating windows
Bioreactor software to facilitate process understanding and transfer
To achieve greater process understanding, drug development companies generate and analyse their cell culture data at a variety of different scales and culture parameters. A common platform for data acquisition, process control and transfer of recipes is a critical part of the equation that ensures data consistency, data integrity and ultimately process transfer success. The BioPAT MFCS SCADA system integrates the functions of a typical supervisory control and data acquisition system with the BioPAT® chemometrics software suite. Chemometrics tools speed up process optimization through facilitating DOE study design and multivariate data analysis. It allows practitioners to compare batches across scales, identify critical process parameters and build the base for optimization and determination of the process design space. During regular production, real time multivariate data analysis with BioPAT®SIMCA online will permit real-time process monitoring, early fault detection and root cause analysis. Operators can respond to the data and make process interventions that ensure the product has the required quality attributes.

Fig. 3: Real-time MVDA with SIMCA online enables monitoring of batches in real-time for quality control, early fault detection and root-cause-analysis.
Multivariate analysis tools will become ever more necessary as the industry incorporates an increasing array of sensors into stainless steel and single-use bioreactors. Measurements such as inline biomass and online glucose can provide engineers with great processing insights. Even more valuable process knowledge comes from understanding how data from these measurements combine. Ultimately, we can use this knowledge to devise more sophisticated, multivariate control strategies that will ensure the consistency of the product harvested from the bioreactor.
Product Quality By Design
Biopharmaceutical companies are increasingly adopting a QbD approach to control strategy development, spurred on by regulators and the promise of greater operating flexibility. Advances in upstream technology will make is easier for these firms to understand the link between their product critical quality attributes (CQAs) and critical process parameters (CPPs). We at Sartorius believe that our QbD enabling platform solutions could actually shorten development timelines and reduce costs. Using our toolbox with QbD methods will be the most efficient means of characterizing processes and will likely avoid the need for rework resulting from taking ill-advised shortcuts.
When engineers design and optimize bioprocesses with production-scale capabilities in mind, robust production will be the result. This, in turn, will build-upon the productivity improvements gained through achieving higher titers by avoiding batch failures and maximizing plant throughput.
By developing an upstream platform of well-aligned, connected and innovative technologies for process development and production, we at Sartorius, are helping the industry deliver robust, high-titer cell culture processes in the shortest possible time. We are providing the technologies that facilitate quality-by-design into upstream processing to provide greater process understanding and safer biological medicines.
References
Bareither, R., Goldfeld, M., Kistler, C., Tait, A., Bargh, N., Oakeshott, R., O’Neill, K., Hoshan, L. & Pollard, D. (2015) Automated disposable small-scale bioreactor for high-throughput process development: implication of the 24 bioreactor array. Pharmaceutical Bioprocessing. June 2015. 3(3). p185-197.
Namdev, K. (2006) Establishing Process Requirements for Scale-up of a High Density Fed-batch Cell Culture. Presented at Culture AAPS Conference, Boston June 22-23, 2006.
