Reducing Risk In mRNA Therapeutic Development
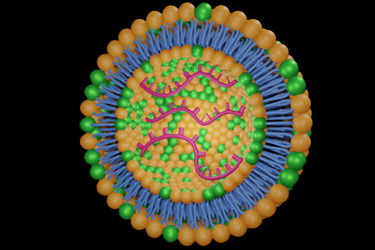
While challenges related to mRNA production, stability, and delivery persist, advancements in these areas are crucial for the successful commercialization of these therapies. Key challenges include optimizing mRNA formats, enhancing lipid nanoparticle delivery systems, and ensuring robust and scalable purification processes. Addressing these complexities, along with supply chain considerations and the development of more stable formulations, will be essential for realizing the full potential of mRNA-based therapeutics.
Ensuring the stability of mRNA molecules throughout the supply chain, from production to administration, is crucial. Additionally, addressing the complexities of lipid nanoparticle formulation and the development of effective purification strategies are essential for achieving desired therapeutic outcomes.
The successful development and commercialization of mRNA therapeutics require a multidisciplinary approach encompassing advancements in mRNA design, delivery systems, manufacturing processes, quality control, and regulatory affairs. Overcoming these challenges will necessitate significant investment in research and development, as well as strong collaborations between academia, industry, and regulatory agencies. Ultimately, the successful translation of mRNA-based therapeutics from the laboratory to the clinic holds the potential to revolutionize patient care and improve human health.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Bioprocess Online? Subscribe today.
