Real-Time Monitoring Of Small Molecule APIs By Light Inducted Fluorescence Sensing For Process Control Applications

Real-time light-induced fluorescence (LIF) has and continues to be an emerging process analytical technology (PAT) for process monitoring and control of auto-fluorescent small molecule pharmaceuticals. LIF’s attraction is attributed to its unique superior sensitivity, low concentration (low dose) detection and rapid response capabilities compared with traditional process analysis approaches such as Raman, near Infrared (NIR), and ultraviolet-visible (UV-Vis) spectroscopies. It can also often afford selective detection without a multivariate or chemometric model as a fluorescent active pharmaceutical ingredient (API) is often contained within a negligible emissive background comprised of non-emissive intermediates and excipients in in-process materials and final dosage formulations, respectively. Moreover, real-time LIF equipped with a suitable in situ probe is also applicable to liquids, solids, semi-solids, and turbid solutions.
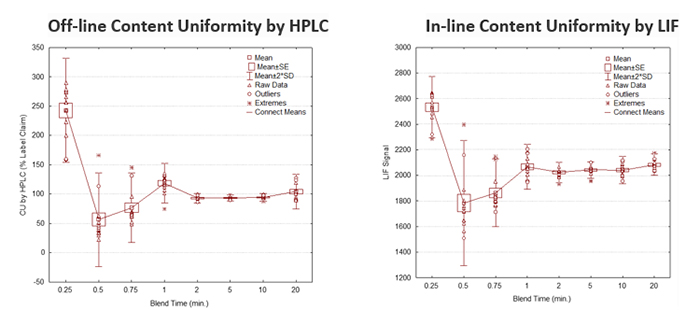
Custom Sensor and Technology’s established photometric LIF sensor has been applied to routine process monitoring and control GMP applications. Our real-time LIF sensor has been used to detect homogeneity endpoints in low-dose pharmaceutical blending applications of solid dose and inhaled formulations (Figure 1).1-5 It has also been utilized for the direct quantitation of solvatomorphs in solid oral dosage forms.6-8 Water quality is also an attractive LIF application.9-12 Numerous applications are probable owing to the plethora of emissive small molecule pharmaceuticals and intermediate.13, 14
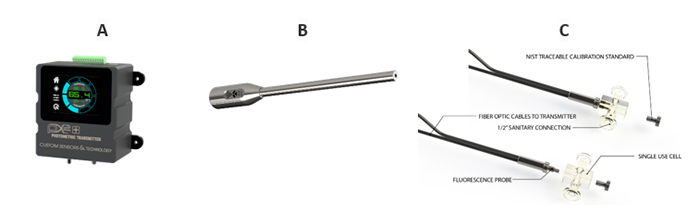
Our LIF sensor product solution can be either supplied with a front surface in-line probe or a single-use flow cell (Figure 2). It can be configured for up to two excitation-emission combinations and comes equipped with suitable solid or liquid performance standards. A photometric configuration enables the full power of LIF by leverages industrial leading LED technology for incident excitation intensity modulation to mitigate photodegradation. This is combined with suitable emission filtering and integrated detection to enable signal response optimization. Consequently, full-spectrum LIF are often overengineered for real-time applications as fluorescence is a broadband spectroscopy well suited for robust solid-state filter-based photometry. Moreover, full-spectrum LIF instrumentation are insufficient as they are often unable to modulate measurement parameters to optimize signal response to thereby meet application criteria. A plethora of small molecule APIs exhibit native fluorescence where our LIF sensor has and continues to find utility.13-15
Summary of LIF applications:
- Continuous blending or blend homogeneity endpoint detection
- Low dose API content in flowing powders
- Direct solvatomorph quantitation
- Water quality
- Solid oral dose, inhaled formulations: liquids, solids, semi-solids, and turbid solutions
References
- Guay, J. M.; Lapointe-Garant, P. P.; Gosselin, R.; Simard, J. S.; Abatzoglou, N., Development of a multivariate light-induced fluorescence (LIF) PAT tool for in-line quantitative analysis of pharmaceutical granules in a V-blender. Eur J Pharm Biopharm 2014, 86 (3), 524-31.
- Igne, B.; Baldasano, C. N.; Airiau, C., Feasibility of Using Light-Induced Fluorescence Spectroscopy for Low-Dose Formulations Monitoring and Control. Journal of Pharmaceutical Innovation 2020.
- Karumanchi, V.; Taylor, M. K.; Ely, K. J.; Stagner, W. C., Monitoring Powder Blend Homogeneity Using Light-Induced Fluorescence. AAPS PharmSciTech 2011, 12 (4), 1031-1037.
- Lai, C. K.; Cooney, C. C., Application of a Fluorescence Sensor for Miniscale On-line Monitoring of Powder Mixing Kinetics. Journal of Pharmaceutical Sciences 2004, 93 (1), 60-70.
- Alves, J. C. L.; Poppi, R. J., Simultaneous determination of acetylsalicylic acid, paracetamol and caffeine using solid-phase molecular fluorescence and parallel factor analysis. Analytica Chimica Acta 2009, 642 (1-2), 212-216.
- Brittain, H. G., Solid-state fluorescence of the trihydrate phases of ampicillin and amoxicillin. AAPS PharmSciTech 2005, 6 (3).
- Brittain, H. G.; Elder, B. J.; Isbester, P. K.; Salerno, A. H., Solid-State Fluorescence Studies of Some Polymorphs of Diflunisal. In Pharmaceutical Research, Springer Netherlands: 2005; Vol. 22, pp 999-1006.
- Geddes, C. D.; Lakowicz, J. R.; Brittain, H. G., Photoluminescence of Pharmaceutical Materials in the Solid State. 4. Fluorescence Studies of Various Solvated and Desolvated Solvatomorphs of Erythromycin A. In Reviews in Fluorescence 2007, Springer New York: 2009; Vol. 2007, pp 379-392.
- Ghervase, L.; Carstea, G.; Savastru, P. D., Laser Induced Fluoresence in Water Quality Assessment. Romanian Reports in Physics 2010, 62, 652–659.
- Research_Water Monitoring organic matter in drinking water systems using fluorescence spectroscopy; 2010.
- Sharikova, A. V.; Killinger, D. K., UV-Laser and LED Fluorescence Detection of Trace Organic Compounds in Drinking Water and Distilled Spirits. InTech: Croatia, 2010.
- Pollard, P. C., Fluorescence instrument for in situ monitoring of viral abundance in water, wastewater and recycled water. Journal of Virological Methods 2012, 181 (1), 97-102.
- Al-Saleh, W. M. Laser and Light induced fluorescence of Some Biofluorophores at Different pH. King Saud University, Riyadh-Saudi Arabia, 2008.
- Albert-Garcia, J. R.; Antón-Fos, G. M.; Duart, M. J.; Lahuerta Zamora, L.; Martínez Calatayud, J., Theoretical prediction of the native fluorescence of pharmaceuticals. Talanta 2009, 79 (2), 412-418.
- Paudel, A.; Raijada, D.; Rantanen, J., Raman spectroscopy in pharmaceutical product design. Advanced Drug Delivery Reviews 2015, 89, 3-20.
