Process Design & Risk Management — A Proactive Approach
By Sandra Wassink, Principal Process Engineer, Pharmatech Associates
The FDA has given us the green light to assess and manage risk earlier in the drug development cycle. Risk assessment is a valuable science-based process, and the Q8 Pharmaceutical Development guidance1 encourages the application of scientific approaches and risk management to the development of a product and manufacturing processes. Addressing risk requires a mindset that serves to determine which elements are critical, how to build more controls into the process, and even how to govern a group of people making assessments. Q9 Quality Risk Management (QRM) guidance2 is an overview of the principles and applications of risk management. The two primary principles of quality risk management are the evaluation of risk to product quality and documentation of the process commensurate with the level of risk.
This article discusses a strategy to implement risk management during the process design stage of process validation for quality product, and to use as a proactive means of addressing quality controls during the subsequent stages of development.
Stages And Steps
As described in FDA’s guidance Process Validation: General Principles and Practices, process design (Stage 1) captures the product development design activities that support and establish the process in preparation for product performance qualification (Stage 2).3 We will focus on Stage 1 and implementing a risk management approach early in the process design using a quality by design (QbD) approach. The goal of this stage is to design a process that is suitable for routine commercial manufacturing that can consistently deliver a product that meets its critical quality attributes.
One of the first steps in the development phase is to draft the quality target product profile (QTPP). It is intended to define product attributes to ensure quality, safety, and efficacy. Examples of product attributes are route of administration, dosage form, bioavailability, strength, and stability.
The next step is to determine the critical quality attributes (CQAs) of the drug substance, raw materials (components), in-process materials, and drug product that have an impact on product quality. The CQAs are determined through use of prior knowledge and experimentation. Selection of an appropriate manufacturing process is important. Control of the process through operational limits and in-process monitoring is essential. Linkage of the material attributes and process parameters to the product CQAs is the beginning of the preliminary risk assessment. Identifying which attributes are critical to meeting product quality and ensuring patient safety are key to the risk assessment process. Once the significant material attributes and process parameters are identified, they can be studied further to gain a higher level of understanding on the impact on product quality. Any attributes that are high risk need to be controlled. Designing a control strategy will ensure that the product will meet the required product quality consistently.
Risk Assessment: Common Approaches
There are many approaches to risk assessment (RA). The goal is to use the appropriate methods and tools for the assessment. Some of the techniques used to construct risk management by organizing information and help decision-making are flow charts, check sheets, process mapping, and cause-and-effect diagrams. Some examples of other methods and tools are failure mode effects analysis (FMEA); failure mode, effects, and critical analysis (FMECA); and hazard operational analysis (HAZOP) that can be used for risk assessment in more complicated processes. It is appropriate to adapt the tools for use with specific areas pertaining to drug product quality. During the early development stage, the risk assessment tool can be simplified, if appropriate, and use a stepwise process. One type of tool is a cause and effect matrix, which identifies risks as high, medium, and low. The steps for this risk assessment tool are the following:
- Step 1: “High level” cause and effect matrix to assess impact of material attributes and process parameters on each CQA
- Identify unit operations that may include critical process parameters (CPPs)
- Step 2: For each unit operation with high and medium risk levels, perform a process parameter risk assessment
- Rely on first principles, historical knowledge, engineering experience
- Consider risk to a CQA, level of control of parameter, risk in scaling up to commercial
- High or medium risk parameters should be considered for further study — design of experiments (DOE)
Preliminary Risk Assessment On Drug Product CQAs
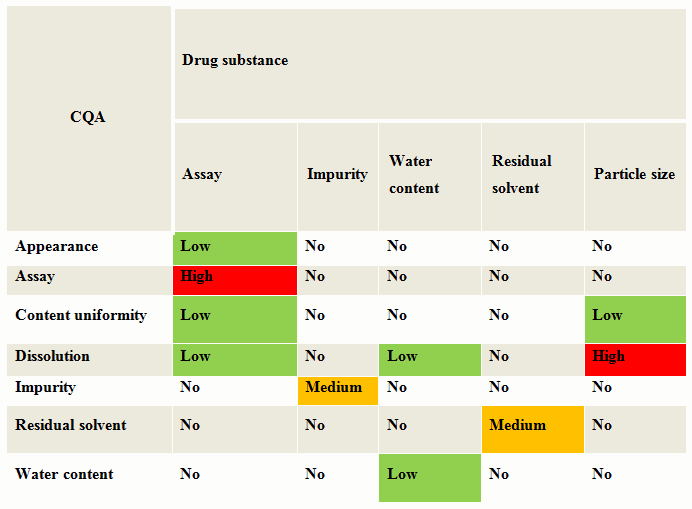
One of the preliminary risk assessments is the evaluation of the attributes of the drug substance (DS) and raw materials to determine if they have an impact on any critical quality attributes (CQAs). Begin by creating a table with the material attributes in a column and the product critical material attributes in a row. For each material attribute, assess if it has an impact on the CQA. This can be ranked as low, medium or high scale.
Table 1 is an example of a cause and effects matrix for drug substance for an oral solid dosage. One example shows how the API particle size influences the assay and release or dissolution rate for an oral dosage form. Any rankings with medium or high would go for further assessment. The drug substance attributes that resulted in a high risk need to be controlled to reduce or eliminate the potential of not meeting the acceptance criteria for the product.

Ready to integrate Quality by Design into your development process? Learn the differences between the old GMP mindset and the new quality systems mindset – and how they both affect our daily operations in the webinar:
Quality by Design (QbD): Making Sense of the ICH Q8, Q9, Q10 Puzzle
Risk control keeps the risk to an acceptable level. The amount of effort that is used for risk control should be proportional to the significance of the risk. The risk assessment should be re-evaluated throughout the development process to ensure there are no changes. If the risk level becomes higher, then risk reduction efforts may need to be implemented. It might also be appropriate to revisit the risk assessment to identify and evaluate any possible changes in risk after implementing a risk reduction process.
Table 1: Cause & Effects Matrix — Oral Solid Dosage
Preliminary Risk Assessment For Process Parameters On CQAs
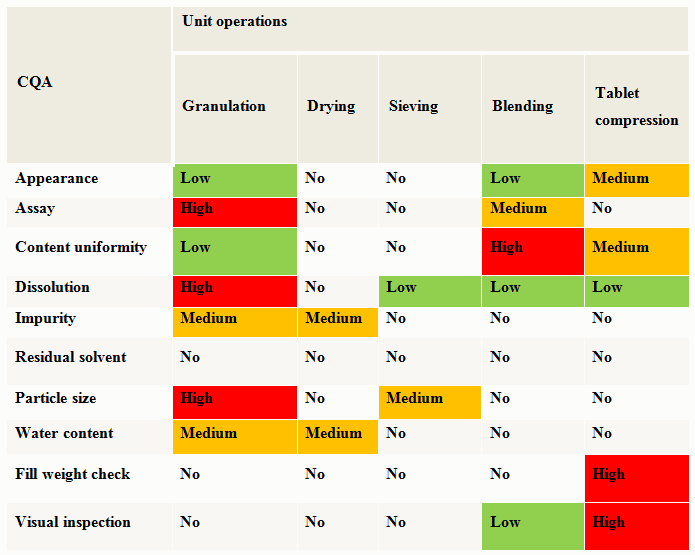
Along with the drug substance and raw material attributes risk assessment, the selection of an appropriate manufacturing process that links material attributes and process parameters to CQAs should be evaluated using a risk assessment tool. A high level evaluation of the unit operation’s impact on the product CQAs is shown as an example in Table 2.
Table 2: Cause & Effects Matrix Example — Oral Solid Dosage (OSD) Uncoated Tablet
Assessing Process Parameter Initial Risk
Unit operations that are ranked as medium or high during the preliminary risk assessment need further evaluation. A detailed assessment of a specific unit operation’s parameters should be performed to determine if a process parameter is critical. A critical process parameter (CPP) is defined as a process parameter whose variability has an impact on a critical quality attribute and therefore should be monitored and controlled to ensure the process produces desired quality. During the manufacturing process development, collection of process monitoring data can provide information to enhance process understanding. Designing experiments to assess the ability of the process to produce reliable product can lead to an understanding of process robustness and is helpful in the risk assessment and risk reduction activities. As additional information is gathered during experimental design activities, the risk assessment should be updated.
Control Strategy Based On Risk Assessment
During product development, a comprehensive approach to a number of control strategy elements will generate process and product understanding and identify sources of variability. In combination with quality risk management and process and product understanding, controls should be implemented to ensure product quality. Control strategy can include the following:
- Control of input material
- Control of unit operations
- In-process or real-time release testing
- Monitoring program
Control strategy can vary depending on the element involved. The risk assessments for the materials and processes can facilitate the identification of measures to control the risks. These controls may be related to attributes, process parameters, in-process controls, and/or procedural or design controls.
Final Considerations
The application of quality risk management and utilization of risk assessments are important during the early part of the development process. Managing risk from the outset helps prioritize what efforts need to be made during process design. The cause and effect matrix is a good and simple risk assessment tool to use when there is limited knowledge. As knowledge increases, the risk assessment should be reevaluated to determine if risk rankings changed. Control strategies for materials and processes should be implemented to ensure product quality.
References:
1. International Conference on Harmonization (ICH) and FDA Guidance for Industry, Q8 (R2) Pharmaceutical Development, Nov. 2009.
2. ICH and FDA Guidance for Industry, Q9 Quality Risk Management, June 2006.
3. FDA Guidance for Industry, Process Validation: General Principles and Practices, Jan. 2011.
About The Author:
 Sandra Wassink, Pharmatech Associates’ principal process engineer, has worked in the regulated life sciences industry for over 30 years. With her technical expertise in QbD and knowledge of global regulatory requirements, she serves as a high-level resource in product and process development and regulatory compliance on numerous client sites and projects for the consultancy. Ms. Wassink holds a B.S. degree in biology with a minor in chemistry from Florida State University.
Sandra Wassink, Pharmatech Associates’ principal process engineer, has worked in the regulated life sciences industry for over 30 years. With her technical expertise in QbD and knowledge of global regulatory requirements, she serves as a high-level resource in product and process development and regulatory compliance on numerous client sites and projects for the consultancy. Ms. Wassink holds a B.S. degree in biology with a minor in chemistry from Florida State University.
