Prioritize Safety And Mitigate Risk In HPAPI Manufacturing
By Dr. Karel Vervisch, Research And Development Manager, Ajinomoto Bio-Pharma Services

Highly potent active pharmaceutical ingredients (HPAPIs) continue to grow in the industry, with current growth figures at around 10.5% per year. As a result, there has been a proportional increase in HPAPI portfolios. Due to their dangerous properties, proper containment and expertise around scale up from lab to production are critical for safe HPAPI manufacturing. Handling these potent compounds requires significant attention to detail and technical knowledge to mitigate risk and maintain technician safety while producing a drug that fulfills customer expectations.
Utilize a Risk-Based Strategy to Produce HPAPIs
Due to their high potency and cytotoxicity, the manufacturing of HPAPIs requires specialized protocols and methodologies. To maintain safety while producing HPAPIs, consider working with a manufacturer who leverages a risk-based approach.
It starts by defining an Occupational Exposure Level (OEL), and if this is not possible, classifying the product into an Occupational Exposure Banding (OEB). OELs and OEBs determine the maximum acceptable level of exposure for personnel, and thus the containment strategy to be used. However, it is important to note that OEB classification systems might differ from company to company.
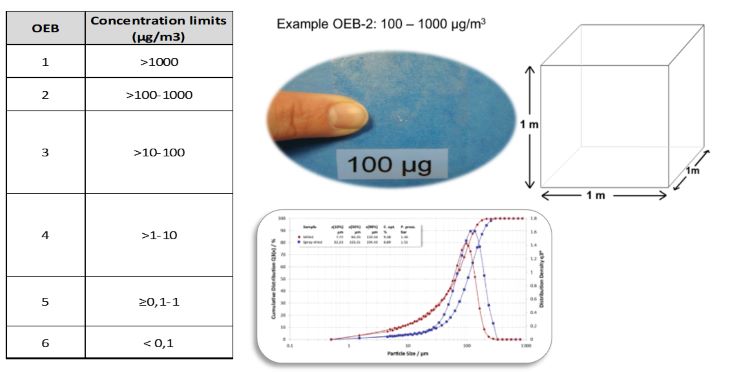
Figure 1 shows the OEB classification used at Aji Bio-Pharma’s sites in Belgium. An HPAPI is characterized by an OEL lower than 10 µg/m³/8h. Since these concentrations are not visible to the human eye and may be difficult to comprehend, we provide a visualization of what 100 µg looks like for training personnel (Figure 1). A clean appearance is not synonymous with safe concentration limits, demonstrating the importance of reliable procedures when handling HPAPIs.
Figure 1. Aji Bio-Pharma’s OEB and a visualization of 100 μg (click to enlarge)
When considering the risk equation (hazard x exposure = risk), hazard is defined by the physical properties of the product, including particle size distribution. The lower the particle size distribution, the better the flowability is and the more likely it is to enter the air. Additionally, some powders are electrically charged, causing them to jump in all directions. Both possibilities pose greater risks.
Exposure is defined by the type of equipment, the physical properties of the product, the unit operation performed, and expertise of the personnel. The unit operation could be stirring a solution in a reactor or milling dry, electrically charged powders. As for the expertise of the people, that includes anyone working with the products or designing the equipment and plants. Depending on the product and other factors, the chances of exposure differ greatly. It is key to have well-informed personnel who will take the correct precautions and are trained to react correctly when an exposure incident happens.
Building Strong Equipment Strategy
Designing safe, low-risk facilities should emphasize three principles:
- Primary containment: reactors, isolation equipment, sampling systems, scavenging units, and process analytics tools
- Secondary containment: the room where primary containment is located, i.e. lab, production warehouse, and sampling areas
- Tertiary containment: personal protective equipment (PPE) worn by employees
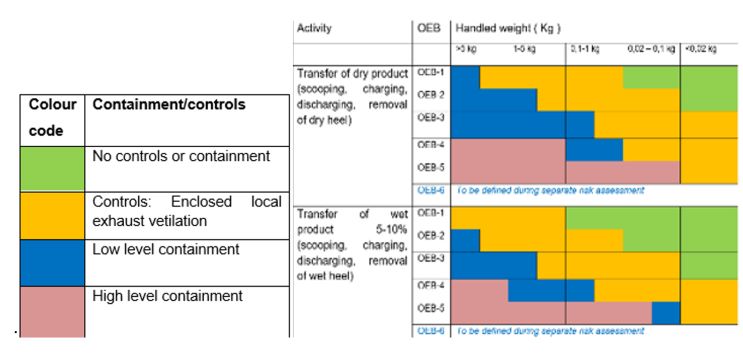
Primary and secondary containment are the predominant layers to protect people and processes against HPAPIs, while tertiary containment provides additional security should something go wrong. The design criteria of these containment levels help ensure increased safety. At Aji Bio-Pharma, we reference internal guidelines to guide our design process. Our global standard is based on the OEB banding of a product, the amount of the product to manipulate, its physical state at the time of manipulation, and the type of handling. Based on those four criteria, different requirements may be needed for primary or secondary containment. For example, when dealing with 200 g of an OEB5 product, the procedure indicates the need for a high level of containment to achieve an occupational exposure below the OEL or otherwise, below the lowest OEB boundary (Figure 2). Therefore, tertiary containment is not needed except as a protection layer in case of incidents.
Figure 2. Color code for containment levels (click to enlarge)
1. Primary Containment
A key component to successful primary containment is collaborating with equipment suppliers on the design. At Aji Bio-Pharma, we work with our suppliers to design equipment well-suited to our specifications. If, for example, we request a filter dryer with fixed or flexible glove boxes, the supplier will provide mock-ups, or wooden constructions of the glove boxes. The personnel who work with the equipment will also consult on the design and its ergonomics.
In doing so, it is important to evaluate all different future unit operations. Some of these unit operations may no longer be considered necessary, including sampling. This is where process analytical techniques (PATs) come in. PATs are effective when dealing with extreme conditions like high-toxicity gases and HPAPIs. PAT use should be factored in at the beginning of equipment design, whether you’re working with a reactor, filter dryer, or other equipment. The manufacturer should provide insight into the following: where to put the PAT probe, if and when to rinse it, and if nitrogen purging or anything else is required. These considerations make for a safe, efficient PAT installation, allowing it to replace sampling when possible.
Material construction is also crucial to consider; since various reactions might occur when manufacturing HPAPIs, chemistry is a major factor. For instance, if you work with azides, you must verify there is no metal involved in your setup. This may be easy for the reactor itself, but when it comes to the coupling of different types of reactors and instruments, it is highly difficult to avoid metals in any part of the equipment setup.
While many designs are made for operations, there are fewer solutions available for cleaning. This is another opportunity for collaboration with suppliers on equipment design. With any engineering project, we consider where the cleaning-in-place will be installed. For instance, if we need to remove the filters, we consider how to rinse the filters with a separate circuit without contaminating the primary circuit, as well as how to measure when it is safe to remove them.
2. Secondary Containment
Secondary containment examines the design of the facility in which the primary containment will be placed and any necessary restrictions. Based on the type of chemistry, there may be additional design restrictions. Primarily though, there will need to be airlocks, HVAC, a cascade of pressure between the different compartments within a building, and continued adherence to quality standards. By utilizing our global standard, we have greater insight into where to use HEPA filtering, the minimum exchange rate of air per type of unit operation, and whether continuous air monitoring is required.
Once designed, all equipment and facilities testing should be conducted to confirm that theory and practice match to ensure safety for our operators, maintenance personnel and lab technicians. Within our ambient air monitoring program, we use the Standardized Measurement of Equipment Particulate Airborne Concentration (SMEPAC) approach.
3. Tertiary Containment
Choosing ideal PPE requires continuously searching for the right tools to make the operators’ burden light and the protection factor high. It is crucial to prioritize ergonomics and easy-to-wear PPE. Some recommended equipment includes an air-fed, powered respirator with an integrated acoustic and visual alarm that indicates if the airflow to the head top is reduced or when the battery charge runs low.
The Impact of High-Quality Personnel
Good equipment is only so effective without hands-on, technically apt teams to operate it. This includes designers, engineers, lab technicians, process operators, and maintenance people. Involving an in-house engineering team for the design and the construction of facilities has proven to be highly beneficial.
At Aji Bio-Pharma, new personnel are guided through a current good manufacturing practice (cGMP) training curriculum that includes standard operating procedures (SOPs) and hands-on critical unit operations and exercises. Our engineering team is engaged from the early process stages of a project. During research and discovery (R&D) and the pilot phase, engineers are designing innovative reactors and considering what a future reactor setup would look like. Based on these results, they provide prototypes to production engineers for larger production units.
Multidisciplinary teamwork is also crucial for SMEPAC exercises and design testing, which can be conducted in a two-step approach. In the first approach, we conduct a dry run of the equipment with lactose. If operators are exposed by way of faulty equipment, there is very low toxicity. If using a filter dryer, we charge, dissolve, crystallize, filter, dry, and discharge the lactose before packing. This serves as practical personnel training in a safe environment where employees can work with new equipment to identify any flaws or difficulties, allowing the equipment to be changed, if necessary, prior to use with HPAPIs. Once all flaws are removed, it is time for the next step.
In the second step, the process is repeated with paracetamol. This time, the ambient air exposure to the operator is monitored by attaching the pump to their PPE. The same pumps are installed in proximity and a bit further away from the location where they do their unit operation, as well as in the corridors outside the secondary containment.
Both steps are guided by an in-house toxicologist and the safety, health, and environment (SHE) department who verifies that everything runs smoothly and prevents false results. For the testing run, paracetamol has a very low detection limit and allows for much more thorough results. By first using lactose and then paracetamol, there is no resulting crossover.
With this protocol, we have proven that in our containment lab we can manage concentration limits of 30 nanograms/m3/8h, which is ranging in the OEB6 levels. SMEPAC testing might also be used to reduce the containment level if there is over processing within the containment unit. High-quality fume hoods (primary containment) can be used safely in the lab to handle OEB4 products, allowing for higher throughput in lab facilities, because there is not a need for secondary containment requirements as with OEB5 products.
Throughout SMEPAC testing, Aji Bio-Pharma collaborates with an external lab that analyzes lactose and paracetamol. We work very closely together to ensure correct interpretation of the results.
What About Process Development?
After establishing safe equipment and working areas, process development begins. Before a project enters our labs, it goes through a request for proposal phase within our internal structure. During that phase, in-house toxicologists assess the OEB classification of the compounds in the project. By knowing the OEB classification of the compound up front, the process chemist is aware of the correct measurements, procedures, and facilities before the start of the project and during process familiarization and development.
An important element of process development is the process safety protocol. Even for HPAPIs, most process safety tests are done in-house by our process safety department, including accelerated rate calorimetry, differential scanning calorimetry, and reaction calorimetry experiments. When dealing with highly potent and explosive properties, a manufacturer may not be equipped for all needed tests. Strong collaboration with suppliers makes it easy to recruit them for these services.
Next, the process chemist begins cleaning validation protocol. At this point, it is critical to develop an analytical method to detect very low concentration levels of highly potent products, including raw materials, intermediates, and end products. When scooping and discharging occur, area sample swapping will verify if the area is clean and ready for release to the next project. These same procedures are conducted in containment labs, pilot labs, and during production to ensure HPAPIs are treated the same in every space.
Once process development is performed in the containment lab, it is time to sample equipment and manufacturing facilities based on predefined destinations from the risk assessment. The areas where the highest amount of highly potent product can be present will be analyzed and must produce an in-spec analytical testing outcome to release the room for new projects. At this stage, manufacturing begins on cGMP production equipment with all defined procedures in place.
Common obstacles during this process are carryovers of previous products via the equipment. HPAPIs require very low levels of carryover and analytical techniques that can detect low levels of highly active compounds. During the setup of a project, a so-called “equipment train” is put together. For each part of this equipment train (e.g., reactor, flexible hoses, fixed transfer lines, filters), the remaining product inside the equipment is quantitatively determined based on cleaning sample analysis. For each specific compound, a maximum acceptable carryover (MACO) is defined upfront by an in-house toxicologist. Only when the accumulated amount is below the maximum acceptable carryover will the equipment and room be released to start the next project.
A Well-Honed HPAPI Approach
To maintain a safe, effective HPAPI risk-mitigation strategy, it is advised to work with a CDMO that conducts continuous risk assessments that are supported by in-house SHE teams. An experienced HPAPI manufacturer has the personnel to witness unit handling from start to finish, and will make productive recommendations on equipment, facilities, and personnel training. Ultimately, conducting continuous safety assessments and executing ambient air monitoring checks to improve design and secure internal and external buy-in produces higher quality HPAPIs and more satisfied customers.
About Aji Bio-Pharma
Ajinomoto Bio-Pharma Services is a CDMO leading the way in research, development, and manufacturing of high-quality chemicals and pharmaceutical products for our customers. Our mission is to improve the health of humankind by being a trustworthy and innovative partner for our customers and our employees. Our contract manufacturing services have three branches: small molecules in Belgium and India, large molecules and aseptic fill/finish on the U.S. west coast, and oligonucleotide and peptide synthesis in Belgium and Japan. We are a diversified, global customer service company focused on quality, safety, and sustainability, who supplies up to 1 billion dosages’ worth of APIs or API compounds per year. Recently, we have conducted major facility expansions across three manufacturing sites to provide increased capabilities for HPAPI production.