PPL Therapeutics Announces Success with Gene Targeting Technology
The new technology, for which PPL has filed patent applications, has immediate utility in some of PPL's existing product development programs, as well as broader implications and uses outside the company's core areas of interest.
PPL's gene targeting technique introduces a gene at a specific place in the chromosomes of livestock cells in culture. Offspring carrying the desired changes are then produced from these cells using nuclear transfer (the technique used by PPL and the Roslin Institute to produce Dolly). Previously, it was only possible to add new genes, but not replace or inactivate existing ones. Also, the site of addition of a gene was previously entirely random and in a different place for every new transgenic animal.
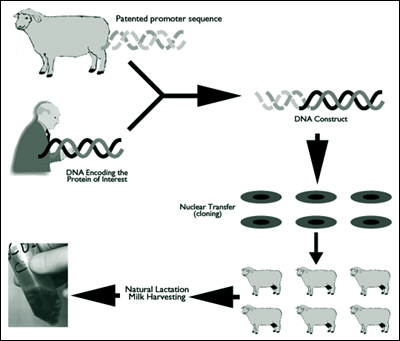
The benefits of this technology are numerous. For example, it will allow a gene for a therapeutic protein to be inserted at a specific chromosomal site selected for its ability to facilitate high expression from any inserted gene. The ability to enhance expression will greatly accelerate PPL's capacity to produce therapeutic and nutritional proteins in the milk of transgenic livestock.
Alan Colman, research director of PPL said: "The range of applications for gene targeting is huge and could bring very real benefits for human healthcare. For PPL, gene targeting and its applications was one of the key reasons for our involvement in nuclear transfer, commonly referred to as cloning, in livestock."
PPL intends to apply the new technology to the replacement of specific genes with their human equivalent. For example, replacing the bovine version of serum albumin with the human form will enable the cost-effective production of human serum albumin (hSA) in the milk of cows. hSA, which is used in the treatment of burns and other traumatic injury, is currently produced from human plasma. PPL believes that cost-effective production in transgenic cows will only be possible if the human albumin gene is substituted for the bovine albumin gene.
The technology might also be applied to situations in which it is necessary to inactivate genes, such as xenotransplantation. The inactivation of a specific gene could lead to animal organs being more readily accepted by the human immune system. Experiments are underway using gene-targeting technology to inactivate the relevant gene in cultured pig cells.
For areas outside its core areas of interest, PPL will seek to license the new technology to other scientists and companies, allowing many people to benefit from its research and also to generate further revenue streams for PPL.
Transgenic technology for producing recombinant proteins was pioneered at the Roslin Institute in Scotland. The technology was licensed and patents assigned to PPL Therapeutics on its foundation in 1987. In 1994, the company was granted a U.S. patent (no. 5,322,775) covering its use of the ß-lactoglobulin (BLG) gene promoter to produce any protein in the milk of any species of transgenic livestock and the subsequent recovery of the protein from the milk.
PPL Therapeutics applies transgenic technology to the production of human proteins for therapeutic and nutritional use.
For more information: Ron James, Managing Director, or Alan Colman, Research Director, PPL Therapeutics, Roslin, Edinburgh EH259PP, U.K. Tel: +44-131-440-4777. Fax: +44-131-440-4888.
