Path To First-In-Human

Unlike traditional CDMOs that stitch services together, Thermo Fisher Scientific connects our capabilities to streamline biologics development through Path to First-in-Human.
Clearing the path: Your journey from preclinical candidate to first-in-human (FiH) trials and beyond demands speed, precision, and confidence at every milestone. Whether you’re advancing toxicology studies, securing your IND/IMPD, or running early-phase clinical research, Path to First-in-Human delivers an integrated, end-to-end solution—uniting development, manufacturing, and clinical execution across one global network in as little as 12 months.*
Key completion milestones for Path to FiH for biologics
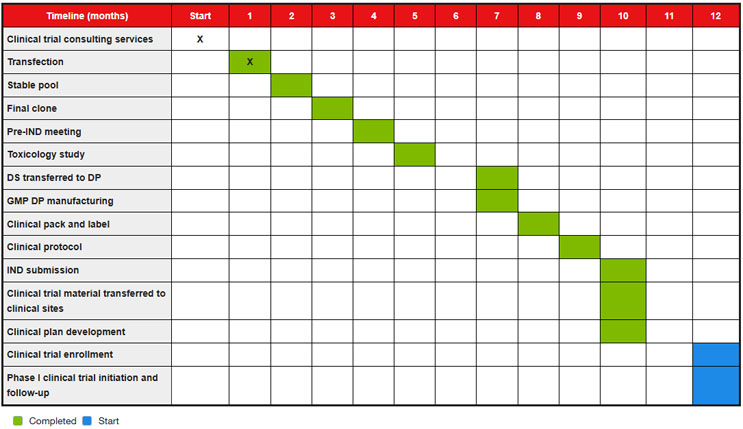
Table 1. Timeline for combined early-phase biologics development services, all completed in-house through Thermo Fisher’s global network, from transfection to Phase I first-in-human clinical trials. * Services shown may be purchased as a combined, integrated offering or individually, based on client preference.
Key benefits of partnering with us for FiH:
- Reduced risk and flexibility: Combined or standalone services, as well as process platforms and custom solutions tailored to your needs
- Integrated services: Thermo Fisher is uniquely positioned to offer bioprocessing, CDMO, and CRO services through a single partner
- Supply security: Raw materials are sourced in-house by leveraging Thermo Fisher’s inventory of high-quality, proven products
- Global expertise: Access to industry experts with deep experience in drug substance and drug product manufacturing, as well as clinical trial management
- Thermo Fisher Financial Solutions: Flexible financing options to support your development and commercialization goals
Why CDMO and CRO integration matters:
- Reduces handoffs and delays by aligning development functions, which can shorten timelines by up to 12 months.1
- Improves coordination and decision-making across teams
- Mitigates risk by proactively identifying roadblocks and enabling greater flexibility to adapt to evolving development needs
1DiMasi, Joseph, Dirks, Abigail, Getz, Kenneth. “The Net Financial Benefits of Single Vendor Integrated CDMO and CRO Drug Development Services.” (2025) Manuscript submitted for peer review and publication. Tufts Center for the Study of Drug Development, Tufts University.
