Not Everyone Loves Surprises: Know Your ADC's Critical Quality Attributes
By Omar Lamm, Product Characterization Technical Specialist, MilliporeSigma, and Martin De Cecco, Principal Scientist for Product Characterization, MilliporeSigma
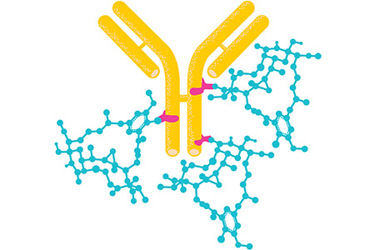
The increased industry-wide focus on cancer, due to its rising patient population and a surge in cancer research, has resulted in an explosion of novel therapeutic drugs to treat this growing global threat. The highly targeted approach of antibody drug conjugates (ADCs), where an antibody is connected to an antitumor cytotoxin via a linker, has been extremely effective in treating various types of cancer. The success of ADCs has now made them the fastest growing class of oncology therapeutics with an expected global market value of $7.5 billion by 2025.¹
Yet, the intrinsic complexity of developing and manufacturing ADCs creates major challenges when trying to bring these life-saving therapies to market. Combatting many of these obstacles requires robust product characterization throughout all phases of development, as small changes can have a big impact, ultimately affecting the success of the final product. Through early identification and a deeper understanding of a drug’s critical quality attributes, you can move to clinical trials faster with the confidence that you are producing a drug with the highest level of safety and quality.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Bioprocess Online? Subscribe today.
