Nontraditional Design Concepts To Accelerate CAR T Processing
By Erich H. Bozenhardt and Herman F. Bozenhardt
 Cell therapies, in particular, chimeric antigen receptor modified T cells (CAR-T), are showing enormous therapeutic promise, with cancer remission rates over 80 percent.1 This alone is forcing cell therapy manufacturing companies to develop strategies to build and scale up production at levels never contemplated before. The processes and facilities for producing these therapies are significantly different from traditional biopharmaceutical production, and there is no template or proven method available. The first round of therapies, now considered “legacy,” is largely being produced using biosafety cabinets and bench-top incubators with multiple open operational steps. In a parallel effort, process development activities are working on the next generation of production processes to reduce or eliminate the open operations and automate cell-based manipulations. To meet the need for these new and evolving therapies, rapidly deployable facilities that work with today’s processes must be adaptable and expandable to the processes of the next five years.
Cell therapies, in particular, chimeric antigen receptor modified T cells (CAR-T), are showing enormous therapeutic promise, with cancer remission rates over 80 percent.1 This alone is forcing cell therapy manufacturing companies to develop strategies to build and scale up production at levels never contemplated before. The processes and facilities for producing these therapies are significantly different from traditional biopharmaceutical production, and there is no template or proven method available. The first round of therapies, now considered “legacy,” is largely being produced using biosafety cabinets and bench-top incubators with multiple open operational steps. In a parallel effort, process development activities are working on the next generation of production processes to reduce or eliminate the open operations and automate cell-based manipulations. To meet the need for these new and evolving therapies, rapidly deployable facilities that work with today’s processes must be adaptable and expandable to the processes of the next five years.
Core Concepts: Modularity And Flexibility
There are many opinions about how these facilities are designed and built, but the concepts of modular processing space and flexibility are universal. Modular spaces are driven by two major aspects: processing capacity and segregation. Increasing production capacity for these autologous (single patient derived) processes follows a scale-out approach. As incremental demands of manufacturing change from clinical trials to commercial production, additional workstations/process trains are added. The complexity and compliance aspects become major risks as uncontrolled expansions occur within one site. Material and product segregation becomes an immediate need and fills multiple functions, reducing risk during open process steps and providing logical organization of products during processing.
Traditional engineering design and building methods can meet the functional needs of these facilities, but the time required by these methods has sent the industry looking for ways to compress the project timeline. The following is a high-level discussion of the process and facility delivery methods.
Methods
Modular cleanroom wall/celling systems like Plascor and AES allow some expediting in the construction of the facility and provide several other improvements over gypsum board construction that have been discussed in other articles. Modular cleanrooms like Extract Technology, Germfree, and Biologics Modular take off-site construction a step further by providing a drop-in-place functional cleanroom, allowing for more paralleling of activities in the project timeline. These build options only address one aspect (the physical building) of the project timeline. The process technology leg (e.g., process development, technology transfer, process validation) and manufacturing leg (e.g., supply train, operator training) will be addressed later.
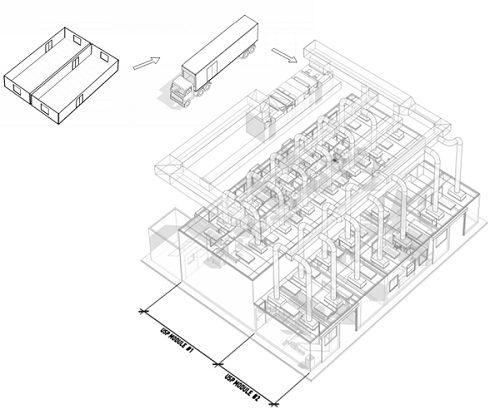
For those early stages in the clinical pipeline, GE has a solution in a combination of its FlexFactory, KUBio, and Fast Trak services. FlexFactory is a GE process equipment platform within a single suite. KUBio is the pre-engineered modular facility that houses FlexFactory suites. Fast Trak is a series of services that include training and process development. The FlexFactory can be delivered on a schedule similar to that of other modular cleanrooms but can reap a major schedule advantage if the process has been developed on the GE platform. Open process steps and/or integration of significant numbers of non-GE systems can be challenging.
G-CON attempts to address the full project in a highly modular design, keeping the compressed schedule of FlexFactory/KUBio, with a higher degree of flexibility. The trade-off is that the owner company has to take a more active role in the facility design.
Case Study 1: In the rapid-fire business of bringing advanced therapies to market, a developer signed a licensing agreement and needed to start making clinical trial materials in under a year. The starting point for this execution was the process fit/capacity analysis. The analysis incorporated the cycle time for a batch, the segregation philosophy, equipment occupancies, and yearly forecast of doses. This set the number of suites, as well as the standard sizes and phasing needs of those suites.
Using the concepts of modularity and flexibility, the first modular suite was operational for training in less than six months (while the future modules’ designs were being refined and built) and the full space started process validation in 11 months from project inception/signing of the licensing agreement. This project used some of the off-site fabrication methods noted above to allow parallel construction activities. Those same off-site fabrication methods also allow “just in time design”/option decisions.
This project demonstrated how standardized modules can enable compressed timelines and facilitate flexibility. Pre-engineered basic facility building blocks allowed for the staged design reviews of typical projects and released the layers in modules based on the level of the review. Whether the process is open biosafety cabinet (BSC)-based, utilizes isolators, or is a closed process, the base building blocks can be the same. This allows process-driven layout decisions to be made along the way, staying just ahead of fabrication. For example, a high-level process capacity analysis identified the number and standard size of suites needed for the facility. This allowed the start of fabrication of the cleanroom modules without detailed room specifications, because they were identical to the previous ones. Follow-up reviews set the size of airlocks, major equipment placement, and low wall returns. Final details were released after the major spatial needs were incorporated, thus allowing compression of the project without sacrificing the ability to fit the facility to the process with a just in time design/decision approach.
The suites were housed in an existing building that was previously a packaging facility. The initial capacity analysis set the number of suites, which determined the floor space required, heating, cooling, and order of magnitude electrical loads for current and future phases. This allowed the work on the hosting facility to start in parallel with the cleanroom and equipment modules a week into the project.
This project also incorporated a phased implementation. A suite and its supporting facility infrastructure were delivered early in the project to facilitate a quick and smooth transition from mechanically complete to operating, by providing a “sandbox” for developing or refining the operating procedures, hands-on training, and operator qualification. The remaining suites delivered within the 11 months provided the capacity to meet the needs of clinical trial material. Future suites for full commercial production were planned, with future suites using the same execution method so that process changes (e.g., fully closed processing or processing in isolators) or operational changes (e.g., open steps in one room and closed steps in another) could be incorporated. This approach allowed compressed timelines and the current construction to be tailored to the process and facilitates future flexibility.
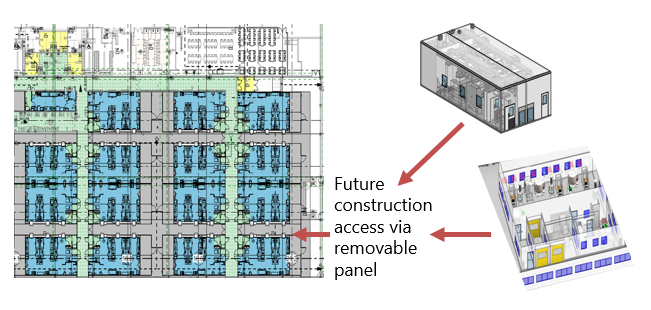
Case Study 2: Another popular approach is to use a contract development and manufacturing organization (CDMO) to carry the product through clinical trials in one facility and then transfer it to a new manufacturing facility for commercial production. Ideally, the CDMO partner has readily available capacity that doesn’t require renovation to start clinical trial batches. While the clinical trials are ongoing, the commercial facility can be designed, built, and qualified. This needs to be carefully staged to mitigate unnecessary capital risk.
In one project, the base of the project was set during the manufacturing of clinical trial material and the early part of the Phase 2 trial. The base included preliminary design, construction needed to facilitate build-out within an existing building shell, and generation of user requirements. This was a traditional execution of the build, but the design was based on the same concepts of modularity and flexibility. With the early part of the schedule not driven to fabricate, design time was taken to pilot several processes and exercise the varying scale of manufacture. Ultimately, for this project, the up-front work to prove the flexibility enabled some of the expended effort to be recaptured when the Phase 2 trial results came in and the lead product was cancelled.
Conclusion
The acceleration of CAR-T processing and capacity requirements demands both nontraditional design concepts and parallel processing, which involve risk-taking. Both of these concepts are traditionally foreign to our industry and have not been part of its skillset. If an organization wants to compete in the highly lucrative business of cell therapy, the mind-set must change, or it will not be successful.
The strategy of modular facility design, simplified engineering, repeatable construction, and standard risk-based qualification is the first major step in meeting these needs. Secondly, incremental, followed by parallel, processing of new process units and modules to produce product is essential to getting the capacity in the market. This may be achieved in-house or via appropriate CDMO facilities. In fact, CDMO facilities are key to having sprint capacity, fallback capacity, and disaster recovery capability. Ultimately, the parallel processing capability lowers an organization’s risk profile.
About The Authors:
 Herman Bozenhardt has 42 years of experience in pharmaceutical, biotechnology, and medical device manufacturing, engineering, and compliance. He is a recognized expert in aseptic filling facilities and systems and has extensive experience in the manufacture of therapeutic biologicals and vaccines. His current consulting work focuses on the areas of aseptic systems, biological manufacturing, and automation/computer systems. He has a B.S. in chemical engineering and an M.S. in system engineering, both from the Polytechnic Institute of Brooklyn. He can be reached via email at hermanbozenhardt@gmail.com and on LinkedIn.
Herman Bozenhardt has 42 years of experience in pharmaceutical, biotechnology, and medical device manufacturing, engineering, and compliance. He is a recognized expert in aseptic filling facilities and systems and has extensive experience in the manufacture of therapeutic biologicals and vaccines. His current consulting work focuses on the areas of aseptic systems, biological manufacturing, and automation/computer systems. He has a B.S. in chemical engineering and an M.S. in system engineering, both from the Polytechnic Institute of Brooklyn. He can be reached via email at hermanbozenhardt@gmail.com and on LinkedIn.
 Erich Bozenhardt is the process manager for Integrated Project Services’ process group in Raleigh, NC. He has 12 years of experience in the biotechnology and aseptic processing business and has led several biological manufacturing projects, including cell therapies, mammalian cell culture, and novel delivery systems. He has a B.S. in chemical engineering and an MBA, both from the University of Delaware. He can be reached via email at ebozenhardt@ipsdb.com and on LinkedIn.
Erich Bozenhardt is the process manager for Integrated Project Services’ process group in Raleigh, NC. He has 12 years of experience in the biotechnology and aseptic processing business and has led several biological manufacturing projects, including cell therapies, mammalian cell culture, and novel delivery systems. He has a B.S. in chemical engineering and an MBA, both from the University of Delaware. He can be reached via email at ebozenhardt@ipsdb.com and on LinkedIn.
References:
