New Microbiological Methods In Bioprocessing
By Ronald A. Rader, BioPlan Associates, Inc.
Testing for evidence of microbial contamination in bioprocessing has a long history. Generally, any detection of viable, replicable microorganisms requires that a contaminated batch or lot be disposed of. Raw materials, reagents, and sterilized stainless-steel equipment must also be tested and confirmed as not being contaminated. All this can be expensive, slow, and burdensome. So suppliers, testing facilities, and regulators have been seeking improvements.
replicable microorganisms requires that a contaminated batch or lot be disposed of. Raw materials, reagents, and sterilized stainless-steel equipment must also be tested and confirmed as not being contaminated. All this can be expensive, slow, and burdensome. So suppliers, testing facilities, and regulators have been seeking improvements.
Microbial testing generally falls into one of three classes:
- Qualitative, broad detection of contamination
- Quantitative estimation of contamination (microbial counts and concentrations)
- Identification of the specific species or strains involved.
Classic methods for microbial detection and quantification in samples include culturing bioprocess fluids on agar-gelled semi-solid and in fluid culture media, with these technologies having been used for well over 100 years. Methods promulgated by the U.S. Pharmacopeia (USP) are the minimum requirements or standards that most regulatory agencies worldwide expect from manufacturers.
Classic Culturing Methods Not Well-suited For Bioprocessing
Because biological manufacturing is time sensitive in terms of how long a batch can be held pending test results, bioprocessing needs new methods for testing. Microbial culturing requires considerable time, generally days, to allow sufficient growth of microbes. Among the worst cases, culture-based detection of mycoplasma contamination and bioburden testing can take about two weeks. This can result in unnecessary discarding of lots/batches when test results are not in hand before product hold time limits expire. Products with short viability and shelf lives are most severely affected, including, in particular, cellular and gene therapies where the products may only have usable lives of hours to a few days.
Most facilities outsource microbial testing, particularly tests supporting release of commercial products, to CROs or CMOs, which can further delay obtaining results with conventional methods. Another complication is that many pathogenic organisms or other unacceptable microbes are not suited to culture-based detection methods. Viruses, which require specific live host cells to infect and replicate, cannot be detected by simple culturing or other methods used with bacteria, fungi, etc. This includes retroviruses hiding within host cell DNA/RNA. Other long-used microbial detection and quantification methods include microscopic examination, ranging from use of common light-based microscopes to desktop or much larger electron microscopy systems.
Types Of Microbial Testing
Most of the microbial testing methods used in bioprocessing are much the same as those used for microbial contamination testing in the food and other industries. Testing for microbial contamination involves multiple types:
- Bioburden/microbial limits: Measure total number of microorganisms to ensure compliance with specifications
- Sterility: Determine presence/absence of detectable microorganisms
- Microbial identification: Identify genus/species of bacteria, viruses, fungi, yeasts, etc.
- Endotoxin: Determine presence of Gram-negative bacteria-excreted toxins
- Mycoplasma: Detect presence of these bacteria with no cell walls
- Water: Measure microorganisms in water, e.g., water for injection, used in bioprocessing and product formulation
- Environmental: Measure microorganisms in the air or on surfaces
Testing Is Increasingly Outsourced
Microbial testing is not considered a core bioprocessing task. A large portion of facilities, if not most, outsource this testing, a practice that is expected to increase. The Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production, published by BioPlan Associates, shows that analytical testing is perhaps the most common outsourcing activity.1 Figure 1 shows selected survey results regarding the activities that are planned to be significantly more outsourced. “Analytical Testing: Other bioassays,” including microbial testing, was by far the number one area cited. There is also a clear trend of increased planning to outsource testing, growing from 18.3 percent in 2011 to 29.6 percent in 2017.
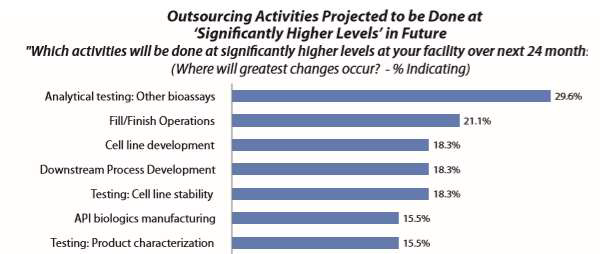
Figure 1: Outsourcing activities projected to be done at significantly higher levels over next 24 months1
RMM Technologies
Rapid microbial methods (RMM) loosely refers to testing methods that enable obtaining results relatively faster than classic methods, including those compendial-specified (see http://rapidmicromethods.com). A variety of RMM technologies have been developed, particularly in the past few decades with the advancement of genetic-based testing methods. Types of RMM technologies include:
- Automated growth-based: Measure the biochemical and physiological parameters that reflect microbe growth (e.g., auto-fluorescence, CO2)
- Cellular component-based: Detect specific microbial cell markers (e.g., ATP bioluminescence)
- Viability-based: Differentiate replicating/living from non-replicating dead microorganisms via stains or fluorescent markers (e.g., flow cytometry)
- Nucleic acid amplification/genetic testing: Detect microbes using nucleic acid/gene amplification (e.g., PCR, gene sequencing)
- Immunological: Use of labeled monoclonal antibodies
- Optical methods: Use of microscopic imaging, increasingly automated
- Spectroscopy-based: Detect microbes using light/optical techniques (e.g., intrinsic fluorescence, Mie scattering), increasingly automated
- Micro-electrical-mechanical systems (MEMS): Use of microarrays, biosensors, Lab-On-A-Chip, nanotechnology, etc. to detect microbial contaminants
- Automated versions of current compendial and other methods
Overall, compared with conventional methods, RMMs are considered to provide the following benefits:
- Faster to perform, saving time (often of utmost importance)
- More accurate
- Provide speedier results, allowing more rapid process turnover and product release
- Result in fewer discarded products and intermediates
- Provide important safety benefits, such as finding more potential hazards
- More suited for in-process, real-time testing
New Testing Options
Overall, sterility, bioburden, and microbial identification testing are generally considered necessary testing where RMM adoption could be beneficial. The alternatives to RMMs include automating existing compendial testing. However, while this could speed the actual testing process, it does not address the need for faster results. Next-generation sequencing (NGS) genetic-based testing methods are candidates to replace much compendial testing. Companies that have developed RMMs, many of which currently offer related products, include Applied Biosciences/Thermo Fisher, DB Diagnostics Systems, bioMerieux, Mettler Toledo, and Rapid Micro Biosystems. Essentially, all the largest and many other CROs and CMOs offer some RMM-based testing services.
At present there remains some industry hesitation to adopt NGS and other RMMs. As with other regulated areas in bioprocessing, issues associated with the quantity of information these new tests can provide create concerns, because there are few guidelines regarding how to interpret the data. For example, NGS can potentially detect any/all bacterial or virus marker gene sequences in a sample, but what does that mean in terms of actual replicable/living microorganisms and whether batches/lots are actually contaminated, including where conventional methods do not find anything?
RMM Adoption Is Slow
Despite offering significant benefits over existing methods, adoption of RMMs has been slow. Much has been written about the regulatory uncertainties, and this may be a primary hurdle. There are disagreements over the roles industry and regulators should play in adopting RMMs; and with few regulations and precedents, uncertainties persist. Official standard testing methods for pharmaceutical products are developed by the USP in the form of compendia. If a method is not included in the USP compendia, the FDA requires validation for that method as equivalent or better than the compendial methods. But a related problem is that the RMMs are simply different, measuring different parameters and providing different information compared with conventional methods. And this has been complicating comparisons and validation.
The FDA has issued some preliminary regulations and welcomes the adoption of RMMs, but some see its actions as insufficient. Officially, RMMs are part of the FDA’s Process Analytical Technology (PAT) initiative under its Pharmaceutical cGMPs for the 21st Century program (launched in 2002). However, to date, most RMM initiatives come from CBER, the biologics division of the FDA. But CDER, the drugs division that regulates more structurally-definable products, e.g., recombinant proteins/antibodies — now the majority of current biopharmaceutical products — has yet to take much action. This is a source for industry frustration. And regulators worldwide are generally lagging way behind the FDA.
Other serious concerns regarding new method adoption include:
- Cost: Equipment is more expensive; adopting and validating new testing is costly
- Conservatism: No actual requirements for adoption means the industry is likely to stick with what it knows
- Time: Adopting RMMs is a multi-year process
- Uncertainties: Especially about how to validate and switch over to RMMs
- Readiness: Most countries are not yet ready to adopt RMMs
- Test sensitivity: Increased sensitivity of testing can result in test results failures where current testing finds no problems
- Lack of staff: Operators are needed who have different expertise
But industry will likely benefit from adopting more RMMs, and with wider adoption these tests may even become compendia accepted. As testing is increasingly outsourced, CROs and CMOs will become critical partners in promoting RMMs. They, along with the biggest manufacturers and cell/gene therapy developers, are potentially the pioneers. Most large biopharmaceutical facilities report they are planning to adopt or are studying adoption of RMMs, but so far few have implemented any. New cellular and gene therapy manufacturers need RMMs the most and will likely be leaders in their adoption, particularly NGS-based testing.
References:
- Langer, E.S., et al., 14th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production, BioPlan Associates, April 2017.
 About The Author:
About The Author:
Ronald A. Rader, senior director of technical research at BioPlan Associates, Inc., has 30+ years of experience as a biotechnology, pharmaceutical, and chemical information specialist/analyst. He was editor/publisher of the Antiviral Agents Bulletin, Federal Bio-Technology Transfer Directory, and BIOPHARMA: Biopharmaceutical Products in the U.S. Market.
