New Class of Mitotic Inhibitors Uncovered in High Throughput Screen
"We are very pleased with this paper," says Tim Mitchison, professor of cell biology and an author on the paper. "For this to come out of an academic lab is unusual, if not unique, at this point."
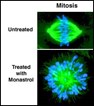
The inhibitor, called monastrol, perturbs the normally bipolar mitotic spindle apparatus in such a way that cells arrest midway through mitosis with a characteristically malformed spindle that looks a bit like an exploding star (see figure).

Steve Haggarty, a graduate student who worked on this project, screened compound libraries using a fast, cheap, and sensitive assay, called cytoblot, which uses an antibody to detect cells suspended in mitosis. Haggarty screened a commercial library containing 16,320 compounds synthesized in Russia, traditionally a powerhouse in chemistry. (Russian chemistry laboratories have begun selling their compounds to supplement shrinking research funds.) In three days, he tested each compound on about 3,000 dividing cells per well of a 384-well plate. With new robots, the institute has since added, this step would now take one day. Researchers led by ICCB fellow Randall King are perfecting a further minituarization step, testing each compound on 1536-well plates, where each well holds 500 cells in five microliters—about a tenth of a rain drop.
One hundred thirty-nine compounds proved to halt cell division. From these, Thomas Mayer, a postdoc in Mitchison's lab, eliminated those that target tubulin, which numbered 53. Many tubulin inhibitors already exist, from colchicine, a research tool, to taxol, a chemotherapeutic drug. Mayer then examined cells microscopically to see how the remaining 86 compounds affected dividing cells.
One jumped out at him. Cells treated with it did not assemble the bipolar spindle that uses microtubules to line up the duplicated chromosomes and then pulls them apart suddenly just before the two daughter cells pinch off. Instead, they formed a monoaster, a central point from which microtubules radiate out, with chromosomes dotted improbably across their middle. Hence the name monastrol.
Based on earlier work in Mitchison's and other labs that showed that monoasters also appear in cells with damage to kinesin, the researcher were able to pinpoint the target for their inhibitor. Using an assay developed by Tarun Kapoor, also a postdoc in Mitchison's lab, for Eg5's ability to move in the presence of its fuel molecule ATP, revealed kinesin as monastrol's target.
The process used by this group represents a reversal of the order drug discovery companies currently favor. Rather than identifying a protein important in a disease and then screening compound libraries against that single target, as does industry, the ICCB scientists screened a library against cell division, a "whole, complex piece of biology," says Mitchison, and then identified the target of the most interesting compound.
It is too early so tell whether monastrol will prove valuable for developing better chemotherapy, says Mitchison. A drug that disrupts spindle components other than microtubules might be more specific than current ones, because all body cells need functioning microtubules while only dividing cells are likely to require complete spindles. Monastrol's relatively weak binding to Eg5—about one order of magnitude below the level at which companies typically start a development program—is counterbalanced by the fact that it already has proven to perform the desired function of stopping mitosis, says Mitchison.
Mayer and Kapoor are using monastrol in ongoing studies of exactly how Eg5 helps build and maintain the mitotic spindle. Mitchison suspects, however, that there is more to Eg5. For example, spindle microtubules are not static; they constantly slide towards the poles. Kapoor is wondering whether Eg5 acts like a "smart glue" that somehow accommodates this movement while keeping the spindle intact. "We can learn a lot more about Eg5, and monastrol will help with that," Mitchison says. "I just love the fact that the first screen landed smack-dab on one of my favorite proteins. Mother Nature was smiling."
For more information: Thomas Mayer, Harvard Medical School, 25 Shattuck St., Boston, MA 02115. Tel: 617-432-1000. Email: Thomas_Mayer@hms.harvard.edu.
