Minimize Contamination Risk With The New Glove Leak Tester

Getinge Wireless Glove Leak Tester (GLT)
In-situ glove testing for complete integrity with traceability
Adhere to stricter regulations
In addition to underlining the importance of selecting isolator gloves with good mechanical and chemical resistance, the internationally recognized guidelines* stipulate that Glove Integrity Testing needs to be included in Standard Operating Procedures – and that gloves must be tested for leakage prior to each production batch. To make this easier, Getinge has developed the new wireless Getinge GLT (Glove Leak Tester) that enables seamless in-situ glove testing.
Getinge’s GLT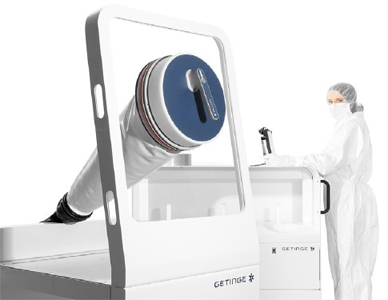
Gloves constitute the most vulnerable link in the containment barrier. Wireless, paperless and pipeless, the new GLT allows for accurate and repeatable testing for glove and sleeve integrity – i.e. perforations not visible to the naked eye.
- Full traceability compliant to FDA 21 CFR part 11 and EU annex 11
- A modern tool compliant to international guidelines*
- Wireless and pipeless (no cables and pipes between the remote head and the main unit)
- Based on pressure decay method with easy application, micro-pumps on board
- HMI available either with external tablet or with onboard version, integrated (through SCADA) for Getinge isolators
![]()
* European Commission, EudraLex, Volume 4, EU Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use, Annex 1, Manufacture of Sterile Medicinal Products, December 2017 (draft for comment) FDA Aseptic Processing Guidance PIC/S Pharma Inspection Convention Cooperation Scheme, Section 9.5.3

