McClintock legacy — first transposase structure solved

"Transposable elements have the potential to remodel genomes and to facilitate the movement of genetic information, such as antibiotic resistance," says Reznikoff, a molecular geneticist.
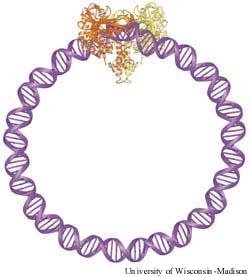
Ribbon diagram of the crystal structure of the Tn5 transposase/DNA complex, with the short pieces of DNA from the crystal structure extended to illustrate how the ends of the transposon bound to the protein/DNA complex are part of a continuous loop. One molecule of transposase is orange and the other is yellow, with the DNA shown in purple.
Previous work on the structure of transposases had focused on the catalytic site of the enzyme, according to Rayment, leaving researchers in the dark on the structure of the entire enzyme or its binding site. Capturing the 3-dimensional structure of the protein-DNA complex has allowed the UW-Madison team to come up with a mechanism of how the enzyme and DNA interact at the molecular level.
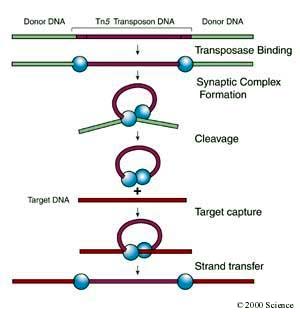
In the first step, individual molecules of transposase (blue spheres in the graphic above) bind to specific sites at the ends of the transposon DNA (purple). In the next step, looping of the transposon DNA results in formation of a synaptic complex that brings the two ends of the transposable element close together. Once the synaptic complex has been formed, the Tn5 transposase cuts the transposon DNA away from the flanking "donor" DNA (green). After cleavage, the Tn5 transposase/DNA complex can move about freely until it encounters and binds to the "target" DNA (red). Through a process called strand transfer the transposase catalyzes insertion of the transposon DNA into the target DNA, completing the transposition process.
These findings have implications for AIDS researchers because the human immunodeficiency virus-1 (HIV-1) uses a process similar to DNA transposition to insert itself into human DNA.
"Just as enzymes called transposases make transposition possible, enzymes called integrases catalyze similar events in retroviruses, including HIV-1," Rayment says. "Researchers have now studied the catalytic core of five different transposases and integrases, and they show remarkable similarity. Therefore, a clear image of one of them provides greater understanding of all similar ones."
To control AIDS, researchers in the pharmaceutical industry are screening compounds that can inhibit HIV-1 integrase, according to Rayment and Reznikoff. Because HIV-1 integrase and Tn5 transposase have similar structures, the Wisconsin scientists believe they now have a model system that can help scientists identify or design compounds effective in controlling HIV-1.
Co-author Douglas Davies worked with Rayment to develop the DNA-enzyme crystals and analyze them using X-ray crystallography. Igor Goryshin, a molecular biologist, worked with Reznikoff in developing, isolating and purifying the transposase.
In 1951, geneticist Barbara McClintock proposed "controlling elements" to explain genetic patterns she observed in corn. Many geneticists were slow to appreciate the importance McClintock's discovery, for which she received a Nobel Prize in 1983. However, researchers have since made remarkable progress in understanding the molecular nature transposable elements.
The research was supported by state funding to the UW-Madison College of Agricultural and Life Sciences, and grants from the National Institute of General Medical Sciences; National Institute of Arthritis and Musculoskeletal and Skin Diseases; the U.S. Department of Energy; and a Vilas Associates Award from the UW-Madison.
For more information: George Gallepp, UW-Madison College of Agriculture and Life Sciences, Agricultural Hall, 1450 Linden Dr., Madison, WI 53706. Tel: 608-262-3636. Email: ggallepp@facstaff.wisc.edu.
