Making Last-Minute Serialization Preparations For The Nov. 2017 DSCSA Deadline
Starting on Nov. 27, 2017, pharmaceutical manufacturers are required to begin marking all saleable units and homogeneous cases of prescription drugs with a unique serial identification code (product identifier), as stipulated by the Drug Supply Chain Security Act. (Editor's note: On June 30, FDA issued new draft guidance pushing back the enforcement deadline for product identifiers to Nov. 27, 2018, though the compliance deadline remains Nov. 27, 2017.) Many drug makers have been preparing for this so-called “serialization” deadline for years by implementing new processes and systems in their internal operations, or by ensuring efficient transfer of serialization data from their contract manufacturing/packaging partners. However, some manufacturers — in particular, smaller or virtual firms — are now engaged in a mad scramble to meet all the requirements of the deadline.
 For some eleventh-hour insights and recommendations regarding the Nov. 27 deadline, we reached out to Bill Webb, senior director of quality, and David Dlugo, senior engineer, of US WorldMeds, a specialty pharmaceutical company based in Louisville, Ky. Webb and Dlugo participated in a panel session on serialization at this year’s FDA/Xavier Health PharmaLink conference, and they both played critical roles in the virtual manufacturer’s serialization implementation. In this Q&A, they share their thoughts on how much drug product to manufacture and package before the deadline, how to select a serialization software provider, and how to prepare for onboarding these providers.
For some eleventh-hour insights and recommendations regarding the Nov. 27 deadline, we reached out to Bill Webb, senior director of quality, and David Dlugo, senior engineer, of US WorldMeds, a specialty pharmaceutical company based in Louisville, Ky. Webb and Dlugo participated in a panel session on serialization at this year’s FDA/Xavier Health PharmaLink conference, and they both played critical roles in the virtual manufacturer’s serialization implementation. In this Q&A, they share their thoughts on how much drug product to manufacture and package before the deadline, how to select a serialization software provider, and how to prepare for onboarding these providers.
What is the impact for producing large quantities of product prior to Nov. 27?
All drug product in the distribution channel prior to Nov. 27, 2017, will need to either be exhausted or returned to the packager and serialized (relabeled) by Nov. 27, 2019.
In anticipation of this, many companies are planning large-quantity manufacturing runs prior to the November deadline. This would allow them extra time to prepare for serialization readiness, which could include selecting and purchasing capital equipment to support serialization, integration with a serialization provider or design of a proprietary serialization tracking database, and redoing packaging artwork/design to accommodate the serialization printed information. Furthermore, companies that have not signed on with a serialization provider are finding that setup could be delayed because of the number of companies ahead of them in the que, as everyone rushes to meet the deadline in these last days.
The key to pre-November large–quantity runs is making sure you only produce enough to support sales, returns, and expiration durations. Companies do not want to disposition excess inventory after Nov. 27, 2019, since reworking product is an expensive endeavor.
Prior to executing the production of the additional quantities, an assessment should be performed to determine the impact on current personnel, as well as on personnel hired specifically to address this immediate need. Once production has been completed, the separation of those temporary personnel could have an adverse effect on remaining team members and/or send the message that serialization is having a negative impact on the business.
What are the key considerations in selecting a third-party serialization partner?
One of the major factors for US WorldMeds in selecting its serialization data management tool was ease of use. As our team reviewed several potential demos, it was evident that some software user interfaces were more intuitive than others. Keeping in mind our end users, who are administers, we wanted the most straightforward, easy-to-navigate, easy-to-view and -filter data tool.
As partners release new versions of their tools, their customers need to be prepared for validation to comply with 21 CFR Part 11. Executing validation activities can be very expensive and time consuming for your IT group. When selecting a tool, make sure you understand if you have the flexibility to revalidate on your schedule. Some serialization partners push their updates several times per year on their schedule, but it is preferable to find a partner that allows you to update based on your company’s validation schedule.
It is important to note that the FDA continues to enforce Part 11 through its new Part 11 inspection and enforcement program. In the last three years alone, the FDA has issued more than 30 warning letters containing Part 11 violations related to validation of software and computer systems.
Also keep in mind that the costs of third-party partners vary tremendously. There is usually a startup fee, an annual maintenance reoccurring cost, and fees for customization/changes. Be sure to compare these costs among providers to find the best fit to your budget. In particular, understand that changes to the database are billed at a high hourly rate, so be sure to define everything up front. And don’t forget hidden costs such as registering your company with GS1, cost of goods sold (COGS) increases at the packager, serialization setup costs at the packager, validation costs, supplier contract changes, and supplier auditing for serialization capabilities.
Prior to initiating your search for a third-party serialization provider, establish a cross-functional team to perform an assessment of the resources needed internally to source, interview, and assign the correct partner for your business. One size does not fit all. Once the serialization partner has been selected and the contracts signed, the internal team should transition to determining the impact on the business and how issues will be elevated to senior leadership for resolution. This team will also act as the source of leadership updates on the status of the serialization implementation strategy, as well as any potential adverse impact on the business.
How should companies prepare prior to engaging a partner?
Before engaging in discussions with a serialization provider, make sure you understand your requirements and business process flows. The key pieces of information needed for this assessment and the development of master data are:
- Global Location Numbers (GLNs)
- Global Trade Item Numbers (GTINs)
- National Drug Code (NDC) number, which includes the labeler code
- Contract manufacturing organizations (CMOs)/packagers and their serialization provider per product
- Third-party logistics (3PL) suppliers and their serialization provider per product
- Key partners/alliances per product
- Packaging configurations and levels (Example: Carton is lowest unit of sale, twenty four cartons in case, and eight cases on a pallet). The carton, bundle, and case should have a GTIN; the pallet may have a serial shipping container code (SSCC).
- Estimated number of batches and quantities per year
- Basic product information such as dose sizes, product name, and brief description
- The date by which each product needs to be serialized
- Each CMO/packager and 3PL completes a serialization questionnaire and provides a quote outlining the cost impacts of adding serialization to the product.
- High-level software validation strategy (Example: Use a third party that is already on your approved supplier list.)
If you fail to gather this information in advance, your kickoff meeting with your selected partner will be more like an information gathering session and increase your implementation timeline.
Follow these steps to prepare for the kickoff meeting:
- Register your company with GS1. The GS1 website makes this step easy to complete. Once you are setup in GS1, you will need to go into the GS1 US Data Hub and create your GTINs and setup your GLNs per product.
- Map out your logistics. Our company used a diagram — see Figure 1. The map shows the relationship and data movement from the NDC holder to the provider to the CMOs and 3PLs. The partner liked this graphical format since it helped them understand the big picture for each product.
- Understand the packaging levels and which level is considered the lowest level of saleable goods. Each level from saleable goods, cartons, bundles, and cases gets a GTIN assigned in GS1. The pallet level typically is assigned an SSCC.

Figure 1
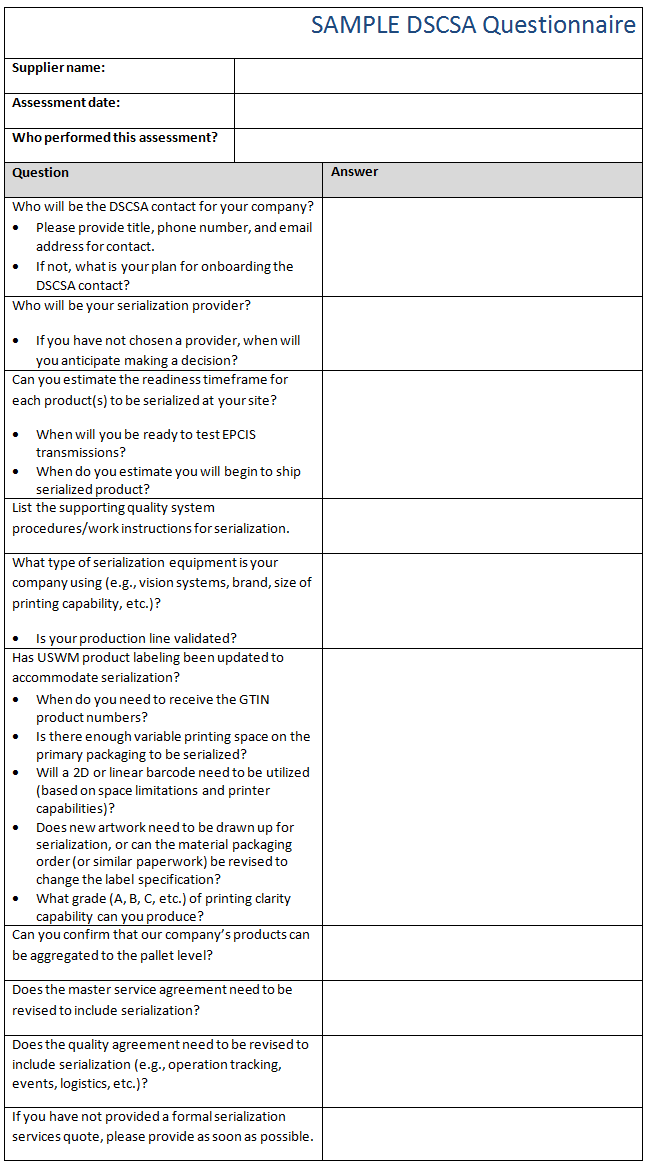
- Collect basic information from your suppliers. Refer to the sample questionnaire below for an example.
- Summarize all this information for your partner. You will find that doing so will make your discussions during the kickoff meeting more straightforward and clear.
The success of all the activities outlined above is predicated on having a well-functioning cross-functional team that has a holistic view of the business and the ongoing requirements of serialization. Without this team, the company can become mired in minor details, become department-focused, experience unnecessary costs and delays, and/or run afoul of regulatory requirements. It is critical that the company provide the necessary resources in a timely manner to accomplish the necessary work in advance. If your company lacks the necessary personnel or specific expertise in-house, it is worth the investment to hire a consultant to protect the reputation and compliance status of your business until the needed resources can be brought on board.