Joint Application Note: Seamless Integration Of Glucose Control Using Raman Spectroscopy In CHO Cell Culture
PAT and QbD guidelines, published by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), reflect the concept that quality cannot be tested into a product but must be deployed throughout process development.
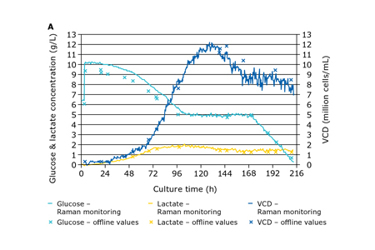
Seamless integration of monitoring into a bioprocess and the application of analytical data are crucial for understanding the process and proactively addressing manufacturing challenges. One of the biggest challenges is in-line monitoring of critical quality attributes (CQA) such as glycosylation that affects the stability, immunogenicity, safety and potency of the biomolecule. Maintaining the glucose concentration at a steady level in the bioreactor is essential to control and optimize the process yield and quality, including glycosylation1,2. Manual sampling and feeding of the bioreactor are costly and time consuming and increase the risk of contamination each time the sterile boundary is penetrated.
This application note describes the use of Procellics™ Raman Analyzer with Bio4C™ PAT Raman Software to monitor glucose levels in a bioreactor and trigger automated addition of feed to maintain the desired concentration.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Bioprocess Online? Subscribe today.
