In-line Monitoring During Downstream Purification
Downstream biopharmaceutical purification unit operations, such as Tangential Flow Filtration (TFF) or ultrafiltration and diafiltration (UF/DF), often necessitate monitoring of total protein or nucleic content in the retentate, permeate or eluate.
The use of at-line or laboratory based full spectrum UV-VIS spectrometer is a common industrial practice for such purposes, to include variable pathlength and microvolume technologies. These approaches, however, require sampling, often involve moving parts (machine based), and significant operator training, which increase GMP risk and cost of ownership. Moreover, separate equipment is often required to enable in-line process monitoring and control capability, thereby substantially increasing capital costs as well as compliance and quality requirements.
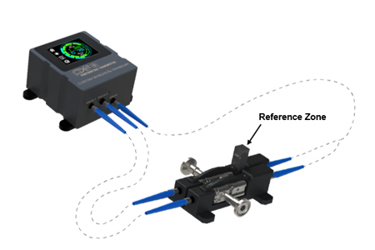
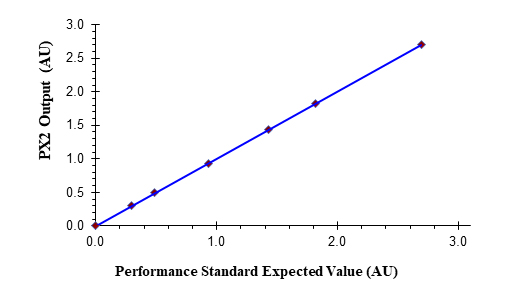
Custom Sensors and Technology manufactures established technology and proven solutions based upon 40 years of product development and industrial experience to enable robust and integrated quality for in-line real-time monitoring across biopharmaceutical unit operations. Our standard solid state dual wavelength process photometer (PX2) is equipped with 260 nm and 280 nm (Figure 1) LEDs or application specific defined wavelengths. Integrated real-time quality is further enabled when a PX2 or alternative full spectrum instrument is combined with our EZ-CAL flow cell (Figure 2). This flow cell affords real-time process monitoring and integrated continuous verification (ongoing compliance) with suitable performance standards. Figure 3 illustrates the response performance of the PX2 and the EX-CAL with commercially available neutral density standards to attenuate absorbance without process disruption. It can also be used to verify wavelengths via a wavelength accuracy standard for applications that leverage a full spectrum spectrometer (UV-Vis, NIR, etc.).

The EZ-CAL solution enables in-line real-time monitoring with integrated quality of monoclonal and polyclonal antibodies, proteins, nucleic acids (mRNA, DNA, etc.) and other target analytes of interest. When the PX2 is utilized in the absorbance mode, the 260/280 nm absorbance ratio affords differentiation between protein and nucleic species where a ratio of 1.7 – 2.0 is indicative of nucleic species whereas a ratio appreciably less than 1.7 is indicative the of proteins, phenols or other species that absorb at 280nm. When utilized in a quantitative or concentration mode, the EZ-CAL can be used to quickly and reliably calibrate the instrument with or without pathlength correction depending upon pathlength specification.
Our combined PX2 and EZ-CAL robust technology is supplied with a qualification package that facilitates readable equipment and method validation. Moreover, a full range of customer services are available to ensure operational continuity to meet and exceed technical and compliance requirements. This cost-effective and integrated quality solution has and continues to enable robust in-line real-time monitoring across biomanufacturing downstream unit operations (Figure 4) across biotherapeutic modalities.

