Immtech International's dicationic molecules effective against fungal and intractable infectious diseases
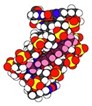
The company's novel business paradigm uses a large university consortium to perform basic research in developing anti-microbial dications.
Table of Contents
Research consortium
In the pipeline
Nailing down the mechanism
 Immtech International Inc., a biopharmaceutical company headquartered in Vernon Hills, Illinois, is setting its sites on fungi and other infectious agents that cause such scourges as tuberculosis, pneumonia and diarrhea. At present, there is inadequate drug therapy for these diseases, and things are likely only to get worse as the world's population of immunocompromised people expands due to the AIDS epidemic. Immtech's core anti-microbial pharmaceutical platform technology is being used to design new drugs with activity against a wide array of infectious organisms. These new compounds work by interfering with enzymes that bind to the minor groove of DNA, and by inhibiting growth, they eventually kill the infectious organisms.
Immtech International Inc., a biopharmaceutical company headquartered in Vernon Hills, Illinois, is setting its sites on fungi and other infectious agents that cause such scourges as tuberculosis, pneumonia and diarrhea. At present, there is inadequate drug therapy for these diseases, and things are likely only to get worse as the world's population of immunocompromised people expands due to the AIDS epidemic. Immtech's core anti-microbial pharmaceutical platform technology is being used to design new drugs with activity against a wide array of infectious organisms. These new compounds work by interfering with enzymes that bind to the minor groove of DNA, and by inhibiting growth, they eventually kill the infectious organisms.
Company researchers have shown that compounds with a dicationic structure (dumbbell-shaped molecules with two positive charges held together by a linker) have activity against fungal, parasitic, bacterial, and viral disease-causing agents. And by playing with the size and nature of the linker, the company has developed drugs that have a common mechanism of action (blocking common enzymes), often giving a single drug broad-based activity against several diseases. In addition, the company's researchers have developed patented drug delivery (prodrug) technology, enabling its positively charged broad-spectrum drugs, typically incapable of crossing cell membranes because of its charge, to be orally active. This prodrug technology has universal applications as an oral delivery method for other classes of positively charged drugs as well.
Research consortium
In tackling these human pathogens, Immtech has chosen to partner with some of the country's leading scientists who have experience working with these organisms. As a practical matter, it relieves the company of the considerable burden of creating laboratories to work on human pathogens, but it also brings the best minds to bear on the problem. Immtech's scientific consortium is led by Richard Tidwell from the University of North Carolina at Chapel Hill (UNC), who has been studying for some 20 years the antimicrobial activity of molecules structurally related to Pentamidine, a dication drug currently in use. Although the drug has significant toxicity, Pentamidine is effective in treating several tropical diseases and PCP, a fungus that grows in lungs and causes pneumonia in patients with compromised immune systems (e.g. people with cancer, HIV, transplants, the elderly, and children).
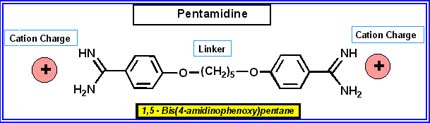
Tidwell and colleagues at UNC discovered that most of Pentamidine's toxicity was caused by metabolic break-down products, rather than the drug itself. This finding led to the design of new compounds with more stable structures that break down in a way that greatly reduces toxicity. These new versions of Pentamidine are less toxic and more efficacious, and have evolved into a new platform technology of compounds that have the potential to treat a wide variety of infectious diseases.
Working with Immtech, Tidwell has established a focused consortium of collaborators to expand on the knowledge and capabilities of dications. Other consortium scientists include John Perfect from Duke University, who provides expertise in fungal diseases, and David Boykin and David Wilson of Georgia State University, who provide expertise in combinatorial chemistry (robotic chemistry) and rational drug design. Scott Franzblau of the University of Illinois in Chicago provides expertise in tuberculosis. In addition to those scientists, Immtech has relationships with Auburn University for its programs in parasitic diseases, molecular biology, and animal husbandry, and London School of Hygiene and Tropical Medicine for tropical diseases.
The evolution of this research has led to second- and third-generation dication molecules with improved potency/toxicity profiles, broad-based applications, and excellent oral bioavailability. These dication compounds have shown excellent activity against the three most prevalent strains of fungus, protozoan parasites, and the normal and drug resistant strains of tuberculosis.
Immtech has an exclusive worldwide licensing agreement for all the dication research performed by the consortium scientists at their universities, including only new compounds developed from the platform. The consortium has developed a large library of over 1,200 compounds that are well categorized, and has the capability to generate thousands of new compounds to expand on the pipeline base. The consortium scientists' expertise and the library of available compounds enable the company to quickly enter into human trials for a wide variety of diseases that address both small and large markets.
In the pipeline
Immtech has two pharmaceutical products entering human Phase I safety trials in the next six months, with one expected to enter human trials in the fourth quarter. DB-289 will be used as a new treatment for Pneumocystis carinii pneumonia, a fungus that causes a severe pneumonia that is life threatening to immune-suppressed patients. The human clinical studies will be conducted under contract with Parexel International, a clinical research organization (CRO), in their Phase I facility in Berlin, Germany. The study is focused on establishing the safety of DB-289 at various doses in normal volunteers. Once safety is established, the company plans to conduct Phase II trials of DB-289 in patients with PCP infections.
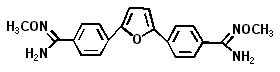
Because of the broad-based activities of DB-289 against many different types of microorganisms, Immtech is discussing conducting a Phase II human trial of DB-289 to treat Trypanosomiasis in sub-Sahara Africa where there is an ongoing epidemic of sleeping sickness.
A second drug, expected to enter human trials in the first quarter of 2001, is DB-075, which will be used as a treatment for Cryptosporidium parvum, a parasite that causes severe diarrhea and is life threatening to immune-suppressed patients. There is not currently a drug available to treat Cryptosporidiosis. In 1993, 100 people died in Milwaukee and 400,000 people were sickened when this microbe contaminated city water supplies. Cryptosporidium is an increasingly problematic disease worldwide due to natural disasters and water contaminated from animal waste found in boundary water.
Nailing down the mechanism
A paper in the October issue of the journal Biochemistry by consortium members Wilson and Tidwell (1) details the application of sophisticated methods to analyze dication interactions, through which they were able to define how dication compounds select specific sites along a DNA sequences. In addition, the researchers identified the mode and bonding characteristics of the compounds' interactions with DNA.
These findings revealed that dication compounds are very accurate when binding to a targeted area of an organism's DNA, giving the compounds a high degree of specificity. As a result, a drug derived from dication compounds has minimal side effects because it preferentially kills microorganisms of the targeted infection. In addition, because of the accuracy of the compound's binding to the targeted DNA, the drug's toxicity is low and the drug is more efficacious.
Determining the target site and binding method for dications is critical to understanding how these compounds work as anti-infective agents for treating diseases, including fungal, parasitic, and tuberculosis diseases.
Steve Thompson, president and CEO of Immtech International Inc., said, "This research provides third-party, peer-reviewed validation of a central claim we have been making about our dication technology—that because of its high specificity, this technology offers an important alternative to the other options currently available on the market and in development. The specificity and mechanism gives Immtech access to dication technology with a wide and deep platform from which many different drugs can be commercialized, a claim that we intend to take fullest advantage of as we continue our development plans."
For more information: T. Stephen Thompson, President and CEO, Immtech International, 150 Fairway Dr., Suite 150, Vernon Hills, IL 60061. Tel: 847-573 0033. Fax: 847-573 8288.
Reference:
- Tanious FA, Ding D, Patrick DA, Bailly C, Tidwell RR, Wilson WD., "Effects of compound structure on carbazole dication-DNA complexes: tests of the minor-groove complex models," Biochemistry. 2000 Oct 3;39(39):12091-101.
Edited by Laura DeFrancesco
Managing Editor, Bioresearch Online
ldefrancesco@bioresearchonline.com
