HRN Targeted Mutation Mouse
 A valuable in vivo model lacking cytochrome P450 activity in the liver
A valuable in vivo model lacking cytochrome P450 activity in the liver
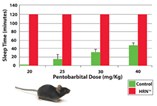
Hudson, NY - Taconic introduces the HRN Mouse, or the Hepatic Reductase Null Mouse, for improved studies in ADME-Tox facilitating drug efficacy screening, lead selection, and lead optimization. Originally developed by Cancer Research UK and commercialized by CXR Biosciences, this conditional, targeted knockout of Por (cytochrome P450 reductase) in the liver results in inactivation of all hepatic cytochrome P450 activity. As the cytochrome P450 system plays a major role in drug metabolism and disposition, this model is very useful for efficacy, bioavailability and ADMET studies. The lack of metabolism increases the circulating levels of compound thereby facilitating bioavailability studies and increasing the chances of demonstrating early in vivo efficacy.
Although cytochrome P450 comprises a large, diverse family of proteins in both humans and animals, they can all be rendered inactive by deleting the gene for P450 reductase, the essential electron donor to all cytochrome P450 isozymes. After hepatic cytochrome P450 activity is eliminated, in vivo drug efficacy can be demonstrated more clearly and with much smaller amounts of valuable lead compounds.
In addition, this model can also provide further information on whether parent compound or metabolite(s) are responsible for observed efficacy or toxicity when compared to wild type mice. The lack of metabolism in the HRN also enables greater exposure to compounds without repeated dosing or the use of constant infusion pumps, even with high clearance compounds. The HRN mouse allows for the true dosing of parent compounds, which may not be otherwise possible. Because of the model's efficiency, researchers can look forward to reducing the number of experiments and animal use in lead selection, thus reducing costs.
SOURCE: Taconic
